Page 183 - Handbook of Battery Materials
P. 183
152 5 Nickel Hydroxides
in the crystallinity of this material has been obtained by hydrothermal treatment
in an aqueous slurry containing NH 4 OH, KOH [18], or NaOH [19]. A method
which produces good crystals has been described by Fievet and Figlarz [20]. The
hydroxide is prepared in two steps. First, an ammonia solution is added to a
nickel nitrate solution at room temperature. The precipitate is washed, and then
◦
hydrothermally treated at 200 C. Another method is to precipitate the hydroxide
◦
by dropwise addition of 3 mol L −1 Ni(NO 3 ) 2 to hot (90 C) 7 mol L −1 KOH with
constant stirring. The precipitate is washed and dried. The Ni(OH) 2 is then
dissolved in 8 mol L −1 NH 4 OH, and the resulting blue solution of Ni(NH 3 ) 6 (OH) 2
is transferred to a desiccator containing concentrated H 2 SO 4 and kept there for
several days. Slow removal of the NH 3 by H 2 SO 4 yields well-formed glassy flakes
of β-Ni(OH) 2 [21]. α-Ni(OH) 2 can be prepared electrochemically, and can be
◦
converted to β-Ni(OH) 2 by heating in 6–9 rnol L −1 KOH at 90 C for 2–3 h [11].
The definitive structural determination of the β-hydroxide is the powder neutron
diffraction work of Greaves and Thomas on β-Ni(OD) 2 [22]. They did neutron
diffraction studies on well-crystallized deuterated Ni(OD) 2 that had been prepared
by a hydrothermal method. They also investigated a high-surface-area Ni(OH) 2
that was prepared by precipitation by addition of KOH to an NiSO 4 solution. The
X-ray and neutron diffraction results indicate that β-Ni(OH) 2 has a brucite C6-type
structure that is isomorphous with the divalent hydroxides of Ca, Mg, Fe, Co, and
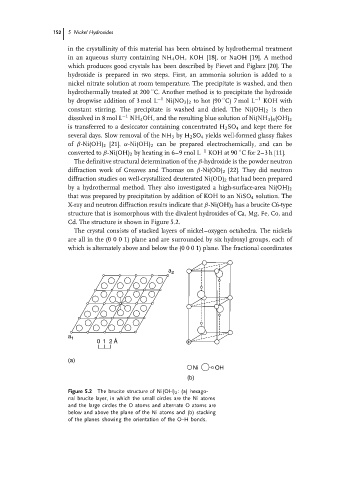
Cd. The structure is shown in Figure 5.2.
The crystal consists of stacked layers of nickel–oxygen octahedra. The nickels
areall in the(0001)planeandaresurrounded bysix hydroxyl groups, eachof
which is alternately above and below the (0 0 0 1) plane. The fractional coordinates
a 2
a 1
0 1 2 Å +
(a)
Ni OH
(b)
Figure 5.2 The brucite structure of Ni(OH) 2 : (a) hexago-
nal brucite layer, in which the small circles are the Ni atoms
and the large circles the O atoms and alternate O atoms are
below and above the plane of the Ni atoms and (b) stacking
of the planes showing the orientation of the O–H bonds.