Page 186 - Handbook of Battery Materials
P. 186
5.3 Solid-State Chemistry of Nickel Hydroxides 155
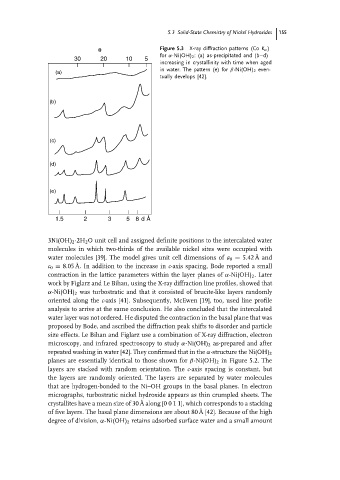
θ Figure 5.3 X-ray diffraction patterns (Co K α )
for α-Ni(OH) 2 : (a) as-precipitated and (b–d)
30 20 10 5
increasing in crystallinity with time when aged
in water. The pattern (e) for β-Ni(OH) 2 even-
(a)
tually develops [42].
(b)
(c)
(d)
(e)
1.5 2 3 5 8 d Å
3Ni(OH) 2 ·2H 2 O unit cell and assigned definite positions to the intercalated water
molecules in which two-thirds of the available nickel sites were occupied with
water molecules [39]. The model gives unit cell dimensions of a 0 = 5.42 ˚ A and
c 0 = 8.05 ˚ A. In addition to the increase in c-axis spacing, Bode reported a small
contraction in the lattice parameters within the layer planes of α-Ni(OH) 2 .Later
work by Figlarz and Le Bihan, using the X-ray diffraction line profiles, showed that
α-Ni(OH) 2 was turbostratic and that it consisted of brucite-like layers randomly
oriented along the c-axis [41]. Subsequently, McEwen [19], too, used line profile
analysis to arrive at the same conclusion. He also concluded that the intercalated
water layer was not ordered. He disputed the contraction in the basal plane that was
proposed by Bode, and ascribed the diffraction peak shifts to disorder and particle
size effects. Le Bihan and Figlarz use a combination of X-ray diffraction, electron
microscopy, and infrared spectroscopy to study α-Ni(OH) 2 as-prepared and after
repeated washing in water [42]. They confirmed that in the α-structure the Ni(OH) 2
planes are essentially identical to those shown for β-Ni(OH) 2 in Figure 5.2. The
layers are stacked with random orientation. The c-axis spacing is constant, but
the layers are randomly oriented. The layers are separated by water molecules
that are hydrogen-bonded to the Ni–OH groups in the basal planes. In electron
micrographs, turbostratic nickel hydroxide appears as thin crumpled sheets. The
crystallites have a mean size of 30 ˚ A along [0 0 1 1], which corresponds to a stacking
of five layers. The basal plane dimensions are about 80 ˚ A [42]. Because of the high
degree of division, α-Ni(OH) 2 retains adsorbed surface water and a small amount