Page 191 - Handbook of Battery Materials
P. 191
160 5 Nickel Hydroxides
α-Ni(OH) 2 . Thus, substitution of 20% of the Ni with these trivalent ions stabilizes
the operation of the electrode in the α/γ cycle in concentrated KOH.
Because of the possibility of applying M¨ ossbauer spectroscopy, the solid-state
chemistry of the Fe-substituted material is the best understood [69, 72, 73].
M¨ ossbauer spectroscopy confirms that the Fe in the pyroaurite type material is
Fe(III). Glemser and co-workers have found that electrochemical oxidation of the
material converts about 30% of the Fe(III) to Fe(IV) [69, 72]. The results were
consistent with a high-spin configuration with the Fe(IV) in FeO 6 octahedra with
O h symmetry. The O h symmetry can only occur if the surrounding NiO 6 octahedra
also have an O h symmetry. Hence, the Fe(IV) ions in the layer must be surrounded
by six NiO 6 octahedra with the Ni in the Ni(IV) state. Delmas and coworkers
found evidence for Fe(IV) in both high- and low-spin states for oxidized materials
prepared by the ‘chemie douce’ method [73]. The difference in the results may be
due to the effect of the platelet size on the pyroaurite structure.
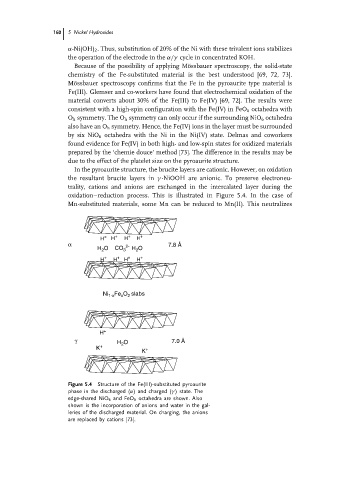
In the pyroaurite structure, the brucite layers are cationic. However, on oxidation
the resultant brucite layers in γ -NiOOH are anionic. To preserve electroneu-
trality, cations and anions are exchanged in the intercalated layer during the
oxidation–reduction process. This is illustrated in Figure 5.4. In the case of
Mn-substituted materials, some Mn can be reduced to Mn(II). This neutralizes
H + H + H + H +
α 7.8 Å
H O CO 3 2- H O
2
2
+ + + +
H H H H
Ni 1-x Fe O slabs
x
2
H +
γ H O 7.0 Å
K + 2 +
K
Figure 5.4 Structure of the Fe(III)-substituted pyroaurite
phase in the discharged (α) and charged (γ ) state. The
edge-shared NiO 6 and FeO 6 octahedra are shown. Also
shown is the incorporation of anions and water in the gal-
leries of the discharged material. On charging, the anions
are replaced by cations [73].