Page 192 - Handbook of Battery Materials
P. 192
5.4 Electrochemical Reactions 161
the charge in the brucite layer; this part of the structure reverts to the β-Ni(OH) 2
structure and the intercalated water and anions are expelled from the lattice. With
this, there is a concomitant irreversible contraction of the interlayer spacing from
7.80 to 4.65 ˚ A [72].
5.4
Electrochemical Reactions
5.4.1
Overall Reaction and Thermodynamics of the Ni(OH) 2 /NiOOH Couple
In normal battery operation, several electrochemical reactions occur on the nickel
hydroxide electrode. These are the redox reactions of the active material, oxygen
evolution, and, in the case of nickel–hydrogen and nickel–metal hydride batteries,
hydrogen oxidation. In addition, there are parasitic reactions such as the corrosion
of nickel current collector materials and the oxidation of organic materials from
separators. The initial reaction in the corrosion process is the conversion of Ni to
Ni(OH) 2 .
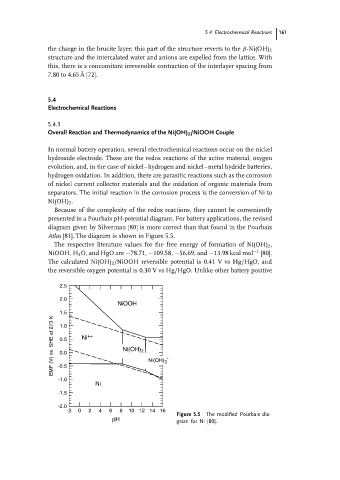
Because of the complexity of the redox reactions, they cannot be conveniently
presented in a Pourbaix pH-potential diagram. For battery applications, the revised
diagram given by Silverman [80] is more correct than that found in the Pourbaix
Atlas [81]. The diagram is shown in Figure 5.5.
The respective literature values for the free energy of formation of Ni(OH) 2 ,
NiOOH, H 2 O, and HgO are −78.71, −109.58, −56.69, and −13.98 kcal mol −1 [80].
The calculated Ni(OH) 2 /NiOOH reversible potential is 0.41 V vs Hg/HgO, and
the reversible oxygen potential is 0.30 V vs Hg/HgO. Unlike other battery positive
2.5
2.0
NiOOH
1.5
EMF (V) vs. SHE at 273 K 0.5 Ni ++ Ni(OH) 2 Ni(OH) 3 -
1.0
0.0
-0.5
-1.0
Ni
-1.5
-2.0
-2 0 2 4 6 8 10 12 14 16
Figure 5.5 The modified Pourbaix dia-
pH gram for Ni [80].