Page 258 - Handbook of Battery Materials
P. 258
228 8 Metallic Negatives
6
5
Mercury content (%) 4
3
2
1
0
1978 1980 1982 1984 1986 1988 1990 1992 1994
Year
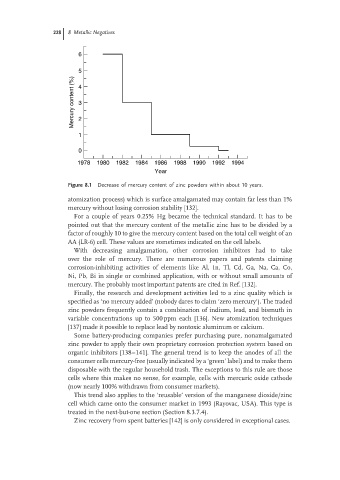
Figure 8.1 Decrease of mercury content of zinc powders within about 10 years.
atomization process) which is surface amalgamated may contain far less than 1%
mercury without losing corrosion stability [132].
For a couple of years 0.25% Hg became the technical standard. It has to be
pointed out that the mercury content of the metallic zinc has to be divided by a
factor of roughly 10 to give the mercury content based on the total cell weight of an
AA (LR-6) cell. These values are sometimes indicated on the cell labels.
With decreasing amalgamation, other corrosion inhibitors had to take
over the role of mercury. There are numerous papers and patents claiming
corrosion-inhibiting activities of elements like Al, In, Tl, Cd, Ga, Na, Ca, Co,
Ni, Pb, Bi in single or combined application, with or without small amounts of
mercury. The probably most important patents are cited in Ref. [132].
Finally, the research and development activities led to a zinc quality which is
specified as ‘no mercury added’ (nobody dares to claim ‘zero mercury’). The traded
zinc powders frequently contain a combination of indium, lead, and bismuth in
variable concentrations up to 500 ppm each [136]. New atomization techniques
[137] made it possible to replace lead by nontoxic aluminum or calcium.
Some battery-producing companies prefer purchasing pure, nonamalgamated
zinc powder to apply their own proprietary corrosion protection system based on
organic inhibitors [138–141]. The general trend is to keep the anodes of all the
consumer cells mercury-free (usually indicated by a ‘green’ label) and to make them
disposable with the regular household trash. The exceptions to this rule are those
cells where this makes no sense, for example, cells with mercuric oxide cathode
(now nearly 100% withdrawn from consumer markets).
This trend also applies to the ‘reusable’ version of the manganese dioxide/zinc
cell which came onto the consumer market in 1993 (Rayovac, USA). This type is
treated in the next-but-one section (Section 8.3.7.4).
Zinc recovery from spent batteries [142] is only considered in exceptional cases.