Page 261 - Handbook of Battery Materials
P. 261
8.3 Battery Anodes (‘Negatives’) 231
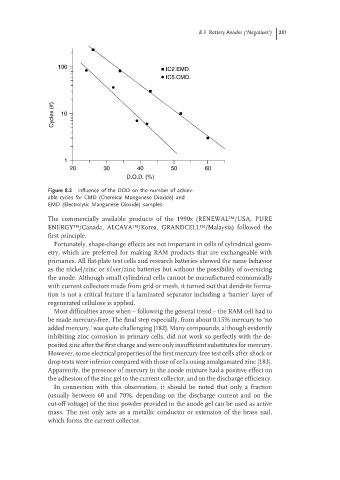
100
IC2.EMD.
IC5.CMD.
Cycles (#) 10
1
20 30 40 50 60
D.O.D. (%)
Figure 8.2 Influence of the DOD on the number of achiev-
able cycles for CMD (Chemical Manganese Dioxide) and
EMD (Electrolytic Manganese Dioxide) samples.
The commercially available products of the 1990s (RENEWAL/USA, PURE
ENERGY/Canada, ALCAVA/Korea, GRANDCELL/Malaysia) followed the
first principle.
Fortunately, shape-change effects are not important in cells of cylindrical geom-
etry, which are preferred for making RAM products that are exchangeable with
primaries. All flat-plate test cells and research batteries showed the same behavior
as the nickel/zinc or silver/zinc batteries but without the possibility of oversizing
the anode. Although small cylindrical cells cannot be manufactured economically
with current collectors made from grid or mesh, it turned out that dendrite forma-
tion is not a critical feature if a laminated separator including a ‘barrier’ layer of
regenerated cellulose is applied.
Most difficulties arose when – following the general trend – the RAM cell had to
be made mercury-free. The final step especially, from about 0.15% mercury to ‘no
added mercury,’ was quite challenging [182]. Many compounds, although evidently
inhibiting zinc corrosion in primary cells, did not work so perfectly with the de-
posited zinc after the first charge and were only insufficient substitutes for mercury.
However, some electrical properties of the first mercury-free test cells after shock or
drop tests were inferior compared with those of cells using amalgamated zinc [183].
Apparently, the presence of mercury in the anode mixture had a positive effect on
the adhesion of the zinc gel to the current collector, and on the discharge efficiency.
In connection with this observation, it should be noted that only a fraction
(usually between 60 and 70%, depending on the discharge current and on the
cut-off voltage) of the zinc powder provided in the anode gel can be used as active
mass. The rest only acts as a metallic conductor or extension of the brass nail,
which forms the current collector.