Page 303 - Handbook of Battery Materials
P. 303
10.2 Physicochemical Properties of Carbon Materials 273
wood (charcoal), coconut and other fruit shells, and low-rank coals. The resulting
carbon is activated by treatment with gas (steam activation) or chemical processing
2
−1
[7]. The end product is a carbon material with high surface area (>1000 m g )
and extensive micropores (pore size <2 nm); these properties were analyzed by
Kaneko et al. [13]. It is this microporosity that contributes to the high adsorption
properties of these carbons.
10.2.2
Chemical Properties
The surface of carbonaceous materials contains numerous chemical complexes
that are formed during the manufacturing step by oxidation or introduced during
post-treatment. The surface complexes are typically chemisorbed oxygen groups
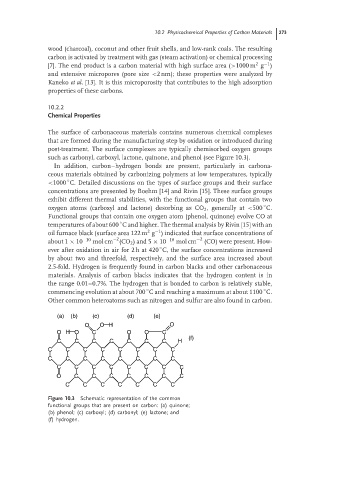
such as carbonyl, carboxyl, lactone, quinone, and phenol (see Figure 10.3).
In addition, carbon–hydrogen bonds are present, particularly in carbona-
ceous materials obtained by carbonizing polymers at low temperatures, typically
◦
<1000 C. Detailed discussions on the types of surface groups and their surface
concentrations are presented by Boehm [14] and Rivin [15]. These surface groups
exhibit different thermal stabilities, with the functional groups that contain two
◦
oxygen atoms (carboxyl and lactone) desorbing as CO 2 , generally at <500 C.
Functional groups that contain one oxygen atom (phenol, quinone) evolve CO at
temperatures of about 600 C and higher. The thermal analysis by Rivin [15] with an
◦
2
−1
oil furnace black (surface area 122 m g ) indicated that surface concentrations of
−2
about 1 × 10 −10 mol cm (CO 2 ) and 5 × 10 −10 mol cm −2 (CO) were present. How-
◦
ever after oxidation in air for 2 h at 420 C, the surface concentrations increased
by about two and threefold, respectively, and the surface area increased about
2.5-fold. Hydrogen is frequently found in carbon blacks and other carbonaceous
materials. Analysis of carbon blacks indicates that the hydrogen content is in
the range 0.01–0.7%. The hydrogen that is bonded to carbon is relatively stable,
◦
◦
commencing evolution at about 700 C and reaching a maximum at about 1100 C.
Other common heteroatoms such as nitrogen and sulfur are also found in carbon.
(a) (b) (c) (d) (e)
O O H O
O H O C O O C
(f)
C C C C C C C H
C C C C C C C C
C C C C C C C C
C C C C C C C C
O C C C C C C C
C C C C C C C
Figure 10.3 Schematic representation of the common
functional groups that are present on carbon: (a) quinone;
(b) phenol; (c) carboxyl; (d) carbonyl; (e) lactone; and
(f) hydrogen.