Page 309 - Handbook of Battery Materials
P. 309
10.3 Electrochemical Behavior 279
2.5
Non-graphitized
Graphitized
2.0
Corrosion rate (%/h) 1.5
1.0
0.5
0.0
0 100 200 300
2
BET surface area (m /g)
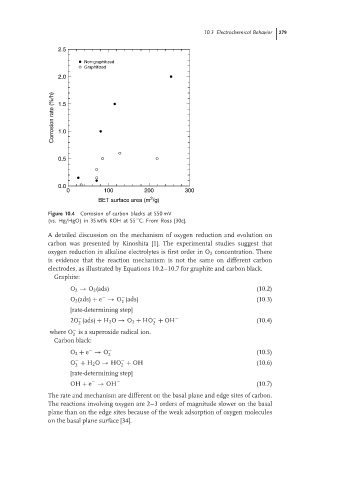
Figure 10.4 Corrosion of carbon blacks at 550 mV
◦
(vs. Hg/HgO) in 35 wt% KOH at 55 C. From Ross [30c].
A detailed discussion on the mechanism of oxygen reduction and evolution on
carbon was presented by Kinoshita [1]. The experimental studies suggest that
oxygen reduction in alkaline electrolytes is first order in O 2 concentration. There
is evidence that the reaction mechanism is not the same on different carbon
electrodes, as illustrated by Equations 10.2–10.7 for graphite and carbon black.
Graphite:
O 2 → O 2 (ads) (10.2)
−
−
O 2 (ads) + e → O (ads) (10.3)
2
[rate-determining step]
−
−
2O (ads) + H 2 O → O 2 + HO + OH − (10.4)
2 2
where O is a superoxide radical ion.
−
2
Carbon black:
−
O 2 + e → O − (10.5)
2
−
−
O + H 2 O → HO + OH (10.6)
2 2
[rate-determining step]
−
OH + e → OH − (10.7)
The rate and mechanism are different on the basal plane and edge sites of carbon.
The reactions involving oxygen are 2–3 orders of magnitude slower on the basal
plane than on the edge sites because of the weak adsorption of oxygen molecules
on the basal plane surface [34].