Page 310 - Handbook of Battery Materials
P. 310
280 10 Carbons
The overpotentials for oxygen reduction and evolution on carbon-based bifunc-
tional air electrodes for rechargeable Zn/air batteries are reduced by utilizing
metal oxide electrocatalysts. Besides enhancing the electrochemical kinetics of the
oxygen reactions, the electrocatalysts serve to reduce the overpotential to minimize
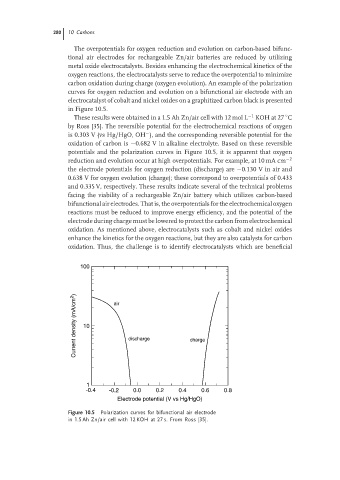
carbon oxidation during charge (oxygen evolution). An example of the polarization
curves for oxygen reduction and evolution on a bifunctional air electrode with an
electrocatalyst of cobalt and nickel oxides on a graphitized carbon black is presented
in Figure 10.5.
◦
These results were obtained in a 1.5 Ah Zn/air cell with 12 mol L −1 KOH at 27 C
by Ross [35]. The reversible potential for the electrochemical reactions of oxygen
−
is 0.303 V (vs Hg/HgO, OH ), and the corresponding reversible potential for the
oxidation of carbon is −0.682 V in alkaline electrolyte. Based on these reversible
potentials and the polarization curves in Figure 10.5, it is apparent that oxygen
reduction and evolution occur at high overpotentials. For example, at 10 mA cm −2
the electrode potentials for oxygen reduction (discharge) are −0.130 V in air and
0.638 V for oxygen evolution (charge); these correspond to overpotentials of 0.433
and 0.335 V, respectively. These results indicate several of the technical problems
facing the viability of a rechargeable Zn/air battery which utilizes carbon-based
bifunctional air electrodes. That is, the overpotentials for the electrochemical oxygen
reactions must be reduced to improve energy efficiency, and the potential of the
electrode during charge must be lowered to protect the carbon from electrochemical
oxidation. As mentioned above, electrocatalysts such as cobalt and nickel oxides
enhance the kinetics for the oxygen reactions, but they are also catalysts for carbon
oxidation. Thus, the challenge is to identify electrocatalysts which are beneficial
100
Current density (mA/cm 2 ) 10 air discharge charge
1
-0.4 -0.2 0.0 0.2 0.4 0.6 0.8
Electrode potential (V vs Hg/HgO)
Figure 10.5 Polarization curves for bifunctional air electrode
in 1.5 Ah Zn/air cell with 12 KOH at 27 s. From Ross [35].