Page 39 - Handbook of Battery Materials
P. 39
1.1 Electrochemical Power Sources 5
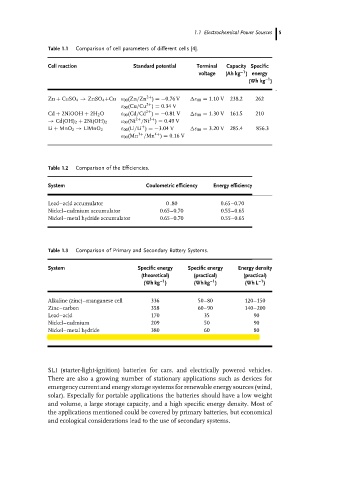
Table 1.1 Comparison of cell parameters of different cells [4].
Cell reaction Standard potential Terminal Capacity Specific
–1
voltage (Ah kg )energy
–1
(Wh kg )
2+
Zn + CuSO 4 → ZnSO 4 +Cu ε 00 (Zn/Zn ) =−0.76 V ε 00 = 1.10 V 238.2 262
2+
ε 00 (Cu/Cu ) = 0.34 V
2+
Cd + 2NiOOH + 2H 2 O ε 00 (Cd/Cd ) =−0.81 V ε 00 = 1.30 V 161.5 210
3+
2+
→ Cd(OH) 2 + 2Ni(OH) 2 ε 00 (Ni /Ni ) = 0.49 V
+
Li + MnO 2 → LiMnO 2 ε 00 (Li/Li ) =−3.04 V ε 00 = 3.20 V 285.4 856.3
ε 00 (Mn /Mn ) = 0.16 V
4+
3+
Table 1.2 Comparison of the Efficiencies.
System Coulometric efficiency Energy efficiency
Lead–acid accumulator 0 .80 0.65–0.70
Nickel–cadmium accumulator 0.65–0.70 0.55–0.65
Nickel–metal hydride accumulator 0.65–0.70 0.55–0.65
Table 1.3 Comparison of Primary and Secondary Battery Systems.
System Specific energy Specific energy Energy density
(theoretical) (practical) (practical)
–1
–1
–1
(Wh kg ) (Wh kg ) (Wh L )
Alkaline (zinc)–manganese cell 336 50–80 120–150
Zinc–carbon 358 60–90 140–200
Lead–acid 170 35 90
Nickel–cadmium 209 50 90
Nickel–metal hydride 380 60 80
Lithium-ion–metal oxide 500–550 150 220
SLI (starter-light-ignition) batteries for cars, and electrically powered vehicles.
There are also a growing number of stationary applications such as devices for
emergency current and energy storage systems for renewable energy sources (wind,
solar). Especially for portable applications the batteries should have a low weight
and volume, a large storage capacity, and a high specific energy density. Most of
the applications mentioned could be covered by primary batteries, but economical
and ecological considerations lead to the use of secondary systems.