Page 42 - Handbook of Battery Materials
P. 42
8 1 Thermodynamics and Mechanistics
10 13
10 11
10 9 7 doped polymers metals
k [Ω -1 cm -1 ] 10 5 3 organic electrolyte sulphuric acid / potassium hydroxide
10
10
10 1 seawater
10 -1
10 -3
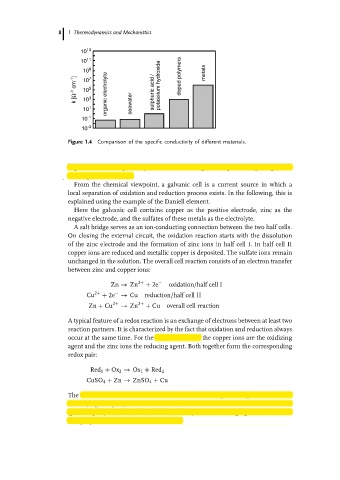
Figure 1.4 Comparison of the specific conductivity of different materials.
depends on its compatibility with the other components, particularly the positive
and negative electrodes.
From the chemical viewpoint, a galvanic cell is a current source in which a
local separation of oxidation and reduction process exists. In the following, this is
explained using the example of the Daniell element.
Here the galvanic cell contains copper as the positive electrode, zinc as the
negative electrode, and the sulfates of these metals as the electrolyte.
A salt bridge serves as an ion-conducting connection between the two half cells.
On closing the external circuit, the oxidation reaction starts with the dissolution
of the zinc electrode and the formation of zinc ions in half cell I. In half cell II
copper ions are reduced and metallic copper is deposited. The sulfate ions remain
unchanged in the solution. The overall cell reaction consists of an electron transfer
between zinc and copper ions:
Zn → Zn 2+ + 2e − oxidation/half cell I
−
Cu 2+ + 2e → Cu reduction/half cell II
Zn + Cu 2+ → Zn 2+ + Cu overall cell reaction
A typical feature of a redox reaction is an exchange of electrons between at least two
reaction partners. It is characterized by the fact that oxidation and reduction always
occur at the same time. For the Daniell element, the copper ions are the oxidizing
agent and the zinc ions the reducing agent. Both together form the corresponding
redox pair:
Red 1 + Ox 2 → Ox 1 + Red 2
CuSO 4 + Zn → ZnSO 4 + Cu
The electrode at which the oxidation dominates during discharge is named the
anode (negative pole), and the other, where the reduction dominates, is the cathode
(positive pole). This nomenclature is valid only for the discharging reaction; for the
charging reaction the names are reversed.