Page 461 - Handbook of Battery Materials
P. 461
434 15 Lithiated Carbons
and medical, and commercial interest is still growing. However, apart from
the rechargeable Li/MnO 2 cell commercialized by Tadiran (Israel) [13–15], the
commercial breakthrough of rechargeable secondary batteries based on metallic
lithium anodes has not been achieved so far. Upon recharge of the anode,
lithium plating occurs simultaneously with lithium corrosion and ‘passivation’
(i.e., formation of SEI). Thus, lithium is deposited as highly dispersed, highly
reactive metal particles. These dendrites are covered with SET films, and therefore
are partially electrochemically inactive. This reduces the efficiency of the lithium
deposition/dissolution process. Moreover, the dendrites grow to filaments upon
cycling, which may short circuit, overheat the cell locally, and cause a disastrous
◦
thermal runaway due to the low melting point of Li (∼180 C) [10, 16–19]. In
contrast, the lithium insertion materials used for the cathode exhibited sufficient
cyclability and safety.
Beginning in the early 1980s [20, 21] metallic lithium was replaced by lithium
insertion materials having a lower standard redox potential than the positive inser-
tion electrode; this resulted in a ‘Li-ion’ or ‘rocking-chair’ cell with both negative
and positive electrodes capable of reversible lithium insertion (see recommended
papers and review papers [7, 10, 22–28]). Various insertion materials have been
proposed for the anode of rechargeable lithium batteries, for example, transition
metal oxides and chalcogenides, carbons, lithium alloys, lithium transition metal
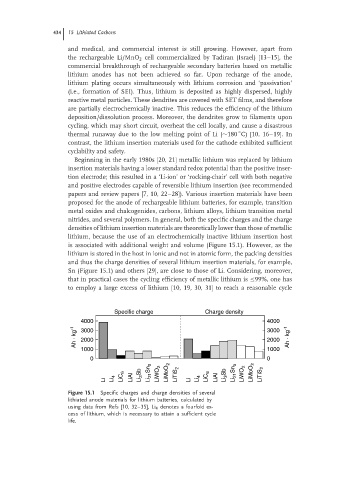
nitrides, and several polymers. In general, both the specific charges and the charge
densities of lithium insertion materials are theoretically lower than those of metallic
lithium, because the use of an electrochemically inactive lithium insertion host
is associated with additional weight and volume (Figure 15.1). However, as the
lithium is stored in the host in ionic and not in atomic form, the packing densities
and thus the charge densities of several lithium insertion materials, for example,
Sn (Figure 15.1) and others [29], are close to those of Li. Considering, moreover,
that in practical cases the cycling efficiency of metallic lithium is ≤99%, one has
to employ a large excess of lithium [10, 19, 30, 31] to reach a reasonable cycle
Specific charge Charge density
4000 4000
Ah · kg -1 3000 3000 Ah · kg -1
2000
2000
1000 1000
0 0
Li Li 4 LiC 6 LiAl Li 3 Sb Li 21 Sn 5 LiWO 2 LiMoO 2 LiTiS 2 Li Li 4 LiC 6 LiAl Li 3 Sb Li 21 Sn 5 LiWO 2 LiMoO 2 LiTiS 2
Figure 15.1 Specific charges and charge densities of several
lithiated anode materials for lithium batteries, calculated by
using data from Refs [10,32–35],Li 4 denotes a fourfold ex-
cess of lithium, which is necessary to attain a sufficient cycle
life.