Page 462 - Handbook of Battery Materials
P. 462
15.1 Introduction 435
life. Therefore, the practical specific charge and the charge density of a secondary
lithium metal electrode are much lower than the theoretical values, almost in the
same order as those of graphite. (More information on the properties of the metallic
lithium anode is given in Part III, Chapter 14.)
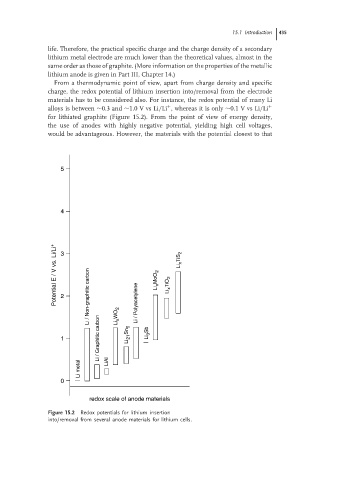
From a thermodynamic point of view, apart from charge density and specific
charge, the redox potential of lithium insertion into/removal from the electrode
materials has to be considered also. For instance, the redox potential of many Li
+
alloys is between ∼0.3 and ∼1.0 V vs Li/Li , whereas it is only ∼0.1 V vs Li/Li +
for lithiated graphite (Figure 15.2). From the point of view of energy density,
the use of anodes with highly negative potential, yielding high cell voltages,
would be advantageous. However, the materials with the potential closest to that
5
4
Potential E / V vs. Li/Li + 3 Li / Non-graphitic carbon Li / Polyacetylene Li x MoO 2 Li x TiO 2 Li x TiS 2
2
1 Li / Graphitic carbon Li x WO 2 Li 21 Sn 5 Li 3 Sb
Li metal LiAl
0
redox scale of anode materials
Figure 15.2 Redox potentials for lithium insertion
into/removal from several anode materials for lithium cells.