Page 467 - Handbook of Battery Materials
P. 467
440 15 Lithiated Carbons
amorphous phase
Crystalline phase
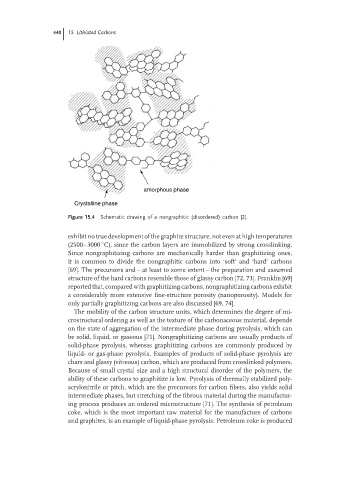
Figure 15.4 Schematic drawing of a nongraphitic (disordered) carbon [2].
exhibit no true development of the graphite structure, not even at high temperatures
◦
(2500–3000 C), since the carbon layers are immobilized by strong crosslinking.
Since nongraphitizing carbons are mechanically harder than graphitizing ones,
it is common to divide the nongraphitic carbons into ‘soft’ and ‘hard’ carbons
[69]. The precursors and – at least to some extent – the preparation and assumed
structure of the hard carbons resemble those of glassy carbon [72, 73]. Franklin [69]
reported that, compared with graphitizing carbons, nongraphitizing carbons exhibit
a considerably more extensive fine-structure porosity (nanoporosity). Models for
only partially graphitizing carbons are also discussed [69, 74].
The mobility of the carbon structure units, which determines the degree of mi-
crostructural ordering as well as the texture of the carbonaceous material, depends
on the state of aggregation of the intermediate phase during pyrolysis, which can
be solid, liquid, or gaseous [71]. Nongraphitizing carbons are usually products of
solid-phase pyrolysis, whereas graphitizing carbons are commonly produced by
liquid- or gas-phase pyrolysis. Examples of products of solid-phase pyrolysis are
chars and glassy (vitreous) carbon, which are produced from crosslinked polymers.
Because of small crystal size and a high structural disorder of the polymers, the
ability of these carbons to graphitize is low. Pyrolysis of thermally stabilized poly-
acrylonitrile or pitch, which are the precursors for carbon fibers, also yields solid
intermediate phases, but stretching of the fibrous material during the manufactur-
ing process produces an ordered microstructure [71]. The synthesis of petroleum
coke, which is the most important raw material for the manufacture of carbons
and graphites, is an example of liquid-phase pyrolysis. Petroleum coke is produced