Page 469 - Handbook of Battery Materials
P. 469
442 15 Lithiated Carbons
Li layer
A C=
0.370
α nm
A
α
A
0.430
(a) nm graphene layer
nm
(b) 0.430 (c) 0.250
nm
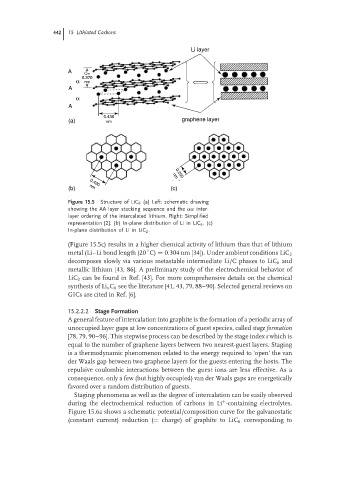
Figure 15.5 Structure of LiC 6 (a) Left: schematic drawing
showing the AA layer stacking sequence and the αα inter-
layer ordering of the intercalated lithium. Right: Simplified
representation [2]. (b) In-plane distribution of Li in LiC 6 .(c)
In-plane distribution of Li in LiC 2 .
(Figure 15.5c) results in a higher chemical activity of lithium than that of lithium
◦
metal (Li–Li bond length (20 C) = 0.304 nm [34]). Under ambient conditions LiC 2
decomposes slowly via various metastable intermediate Li/C phases to LiC 6 and
metallic lithium [43, 86]. A preliminary study of the electrochemical behavior of
LiC 2 can be found in Ref. [43]. For more comprehensive details on the chemical
synthesis of Li x C 6 see the literature [41, 43, 79, 88–90]. Selected general reviews on
GICs are cited in Ref. [6].
15.2.2.2 Stage Formation
A general feature of intercalation into graphite is the formation of a periodic array of
unoccupied layer gaps at low concentrations of guest species, called stage formation
[78, 79, 90–96]. This stepwise process can be described by the stage index s which is
equal to the number of graphene layers between two nearest-guest layers. Staging
is a thermodynamic phenomenon related to the energy required to ‘open’ the van
der Waals gap between two graphene layers for the guests entering the hosts. The
repulsive coulombic interactions between the guest ions are less effective. As a
consequence, only a few (but highly occupied) van der Waals gaps are energetically
favored over a random distribution of guests.
Staging phenomena as well as the degree of intercalation can be easily observed
+
during the electrochemical reduction of carbons in Li -containing electrolytes.
Figure 15.6a shows a schematic potential/composition curve for the galvanostatic
(constant current) reduction (= charge) of graphite to LiC 6 corresponding to