Page 474 - Handbook of Battery Materials
P. 474
15.2 Graphitic and Nongraphitic Carbons 447
15.2.3
Li x C 6 vs Li x (solv) y C n
Apart from reactions with the electrolyte at the carbon surface, the irreversible
specific charge is furthermore strongly affected by the possible co-intercalation of
polar solvent molecules between the graphene layers of highly graphitic matrices
[136]. This so-called ‘solvated intercalation reaction’ depends (i) on the crystallinity
and the morphology of the parent carbonaceous material, which will be discussed
in Section 15.2.4 and (ii) on the composition of the electrolyte, which is discussed
in this section.
Whereas the electrochemical decomposition of propylene carbonate (PC) on
+
graphite electrodes at potentials between ∼1and ∼0.8 V vs Li/Li was already
reported in 1970 [137], it took about four years to find out that this reaction
is accompanied by a partially reversible electrochemical intercalation of solvated
+
lithium ions, Li (solv) y , into the graphite host [63]. In general, the intercalation
+
of Li (and other alkali-metal) ions from electrolytes with organic donor solvents
into fairly crystalline graphitic carbons quite often yields solvated (ternary) lithiated
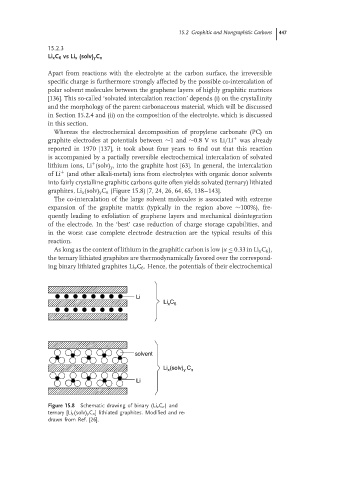
graphites, Li x (solv) y C n (Figure 15.8) [7, 24, 26, 64, 65, 138–143].
The co-intercalation of the large solvent molecules is associated with extreme
expansion of the graphite matrix (typically in the region above ∼100%), fre-
quently leading to exfoliation of graphene layers and mechanical disintegration
of the electrode. In the ‘best’ case reduction of charge storage capabilities, and
in the worst case complete electrode destruction are the typical results of this
reaction.
As long as the content of lithium in the graphitic carbon is low (x ≤ 0.33 in Li x C 6 ),
the ternary lithiated graphites are thermodynamically favored over the correspond-
ing binary lithiated graphites Li x C 6 . Hence, the potentials of their electrochemical
Li
Li C
x 6
solvent
Li (solv) C n
y
x
Li
Figure 15.8 Schematic drawing of binary (Li x C n )and
ternary [Li x (solv) y C n ] lithiated graphites. Modified and re-
drawn from Ref. [26].