Page 476 - Handbook of Battery Materials
P. 476
15.2 Graphitic and Nongraphitic Carbons 449
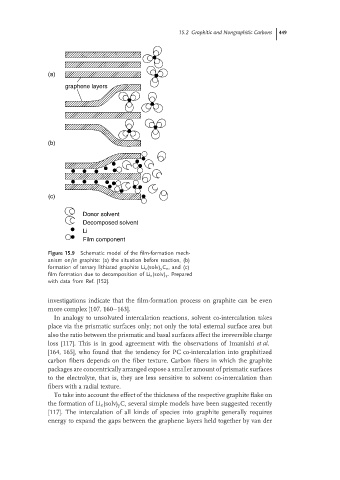
(a)
graphene layers
(b)
(c)
Donor solvent
Decomposed solvent
Li
Film component
Figure 15.9 Schematic model of the film-formation mech-
anism on/in graphite: (a) the situation before reaction, (b)
formation of ternary lithiated graphite Li x (solv) y C n ,and (c)
film formation due to decomposition of Li x (solv) y . Prepared
with data from Ref. [152].
investigations indicate that the film-formation process on graphite can be even
more complex [107, 160–163].
In analogy to unsolvated intercalation reactions, solvent co-intercalation takes
place via the prismatic surfaces only; not only the total external surface area but
also the ratio between the prismatic and basal surfaces affect the irreversible charge
loss [117]. This is in good agreement with the observations of Imanishi et al.
[164, 165], who found that the tendency for PC co-intercalation into graphitized
carbon fibers depends on the fiber texture. Carbon fibers in which the graphite
packages are concentrically arranged expose a smaller amount of prismatic surfaces
to the electrolyte, that is, they are less sensitive to solvent co-intercalation than
fibers with a radial texture.
To take into account the effect of the thickness of the respective graphite flake on
the formation of Li x (solv) y C, several simple models have been suggested recently
[117]. The intercalation of all kinds of species into graphite generally requires
energy to expand the gaps between the graphene layers held together by van der