Page 481 - Handbook of Battery Materials
P. 481
454 15 Lithiated Carbons
B B
0.400 nm
A B B A B B
B B A B B
A BB A BB
B A B B A B
A BB A BB
B B A B B
A B B A B B B B
0.250 nm
A intercalated Li
B B covalent Li molecule
2
(a) (b)
Li located between graphene layers
S S
S C C S S S
C C
S C C
C
S C S
C
C
S S
S S-site
Li located at the Li located at the C C-site
surface of edges of
graphite particles graphene layers
(d)
carbon layer
(c) lithium
(e) intercalated Li Li in cavity
(f) lithium
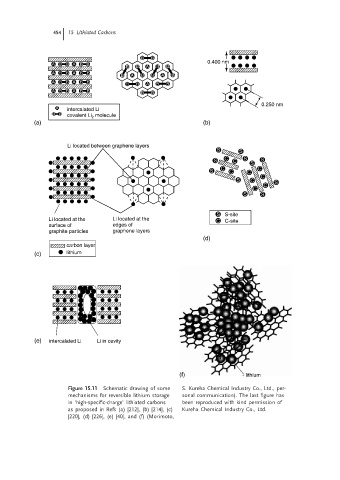
Figure 15.11 Schematic drawing of some S. Kureha Chemical Industry Co., Ltd., per-
mechanisms for reversible lithium storage sonal communication). The last figure has
in ‘high-specific-charge’ lithiated carbons been reproduced with kind permission of
as proposed in Refs (a) [212], (b) [214], (c) Kureha Chemical Industry Co., Ltd.
[220], (d) [226], (e) [40], and (f) (Morimoto,