Page 485 - Handbook of Battery Materials
P. 485
458 15 Lithiated Carbons
3
2.5 2
E / V vs. Li/Li + 1.5 1
0.5
0
0 200 400 600
C / Ah·kg -1
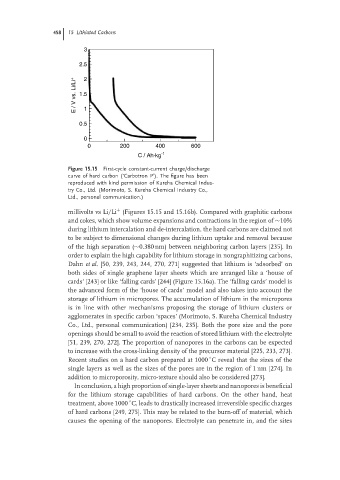
Figure 15.15 First-cycle constant-current charge/discharge
curve of hard carbon (‘Carbotron P’). The figure has been
reproduced with kind permission of Kureha Chemical Indus-
try Co., Ltd. (Morimoto, S. Kureha Chemical Industry Co.,
Ltd., personal communication.)
millivolts vs Li/Li (Figures 15.15 and 15.16b). Compared with graphitic carbons
+
and cokes, which show volume expansions and contractions in the region of ∼10%
during lithium intercalation and de-intercalation, the hard carbons are claimed not
to be subject to dimensional changes during lithium uptake and removal because
of the high separation (∼0.380 nm) between neighboring carbon layers [235]. In
order to explain the high capability for lithium storage in nongraphitizing carbons,
Dahn et al. [50, 239, 243, 244, 270, 271] suggested that lithium is ‘adsorbed’ on
both sides of single graphene layer sheets which are arranged like a ‘house of
cards’ [243] or like ‘falling cards’ [244] (Figure 15.16a). The ‘falling cards’ model is
the advanced form of the ‘house of cards’ model and also takes into account the
storage of lithium in micropores. The accumulation of lithium in the micropores
is in line with other mechanisms proposing the storage of lithium clusters or
agglomerates in specific carbon ‘spaces’ (Morimoto, S. Kureha Chemical Industry
Co., Ltd., personal communication) [234, 235]. Both the pore size and the pore
openings should be small to avoid the reaction of stored lithium with the electrolyte
[51, 239, 270, 272]. The proportion of nanopores in the carbons can be expected
to increase with the cross-linking density of the precursor material [225, 233, 273].
◦
Recent studies on a hard carbon prepared at 1000 C reveal that the sizes of the
single layers as well as the sizes of the pores are in the region of 1 nm [274]. In
addition to microporosity, micro-texture should also be considered [273].
In conclusion, a high proportion of single-layer sheets and nanopores is beneficial
for the lithium storage capabilities of hard carbons. On the other hand, heat
◦
treatment, above 1000 C, leads to drastically increased irreversible specific charges
of hard carbons [249, 275]. This may be related to the burn-off of material, which
causes the opening of the nanopores. Electrolyte can penetrate in, and the sites