Page 490 - Handbook of Battery Materials
P. 490
15.3 Lithiated Carbons vs Competing Anode Materials 463
400 STALION
Li-lon cell
(TOC-anode)
300 common Li-lon cell
Energy density / Wh·L -1 200 Ni-MH cell
(carbon anode)
100
Ni-Cd cell
0
0 50 100 150
Specific energy / Wh·kg -1
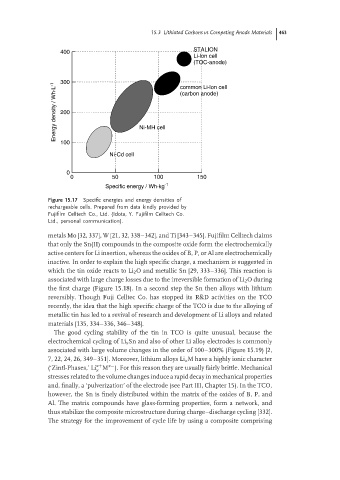
Figure 15.17 Specific energies and energy densities of
rechargeable cells. Prepared from data kindly provided by
Fujifilm Celltech Co., Ltd. (Idota, Y. Fujifilm Celltech Co.
Ltd., personal communication).
metals Mo [32, 337], W [21, 32, 338–342], and Ti [343–345]. Fujifilm Celltech claims
that only the Sn(II) compounds in the composite oxide form the electrochemically
active centers for Li insertion, whereas the oxides of B, P, or Al are electrochemically
inactive. In order to explain the high specific charge, a mechanism is suggested in
which the tin oxide reacts to Li 2 O and metallic Sn [29, 333–336]. This reaction is
associated with large charge losses due to the irreversible formation of Li 2 Oduring
the first charge (Figure 15.18). In a second step the Sn then alloys with lithium
reversibly. Though Fuji Celltec Co. has stopped its R&D activities on the TCO
recently, the idea that the high specific charge of the TCO is due to the alloying of
metallic tin has led to a revival of research and development of Li alloys and related
materials [135, 334–336, 346–348].
The good cycling stability of the tin in TCO is quite unusual, because the
electrochemical cycling of Li x Sn and also of other Li alloy electrodes is commonly
associated with large volume changes in the order of 100–300% (Figure 15.19) [2,
7, 22, 24, 26, 349–351]. Moreover, lithium alloys Li x M have a highly ionic character
x−
x+
(‘Zintl-Phases,’ Li M ). For this reason they are usually fairly brittle. Mechanical
x
stresses related to the volume changes induce a rapid decay in mechanical properties
and, finally, a ‘pulverization’ of the electrode (see Part III, Chapter 15). In the TCO,
however, the Sn is finely distributed within the matrix of the oxides of B, P, and
Al. The matrix compounds have glass-forming properties, form a network, and
thus stabilize the composite microstructure during charge–discharge cycling [332].
The strategy for the improvement of cycle life by using a composite comprising