Page 486 - Handbook of Battery Materials
P. 486
15.2 Graphitic and Nongraphitic Carbons 459
Li in nanopores
Li adsorbed on
carbon layer surfaces
carbon layer
lithium
2.00
1.60
E / V vs. Li/Li + 1.20 Discharge
0.80
0.40
Charge
0.00
0 200 400 600 800
Ah·kg -1
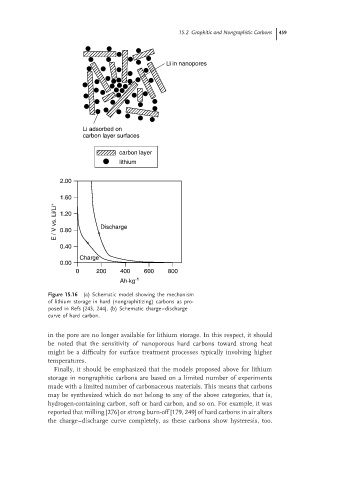
Figure 15.16 (a) Schematic model showing the mechanism
of lithium storage in hard (nongraphitizing) carbons as pro-
posed in Refs [243, 244]. (b) Schematic charge–discharge
curve of hard carbon.
in the pore are no longer available for lithium storage. In this respect, it should
be noted that the sensitivity of nanoporous hard carbons toward strong heat
might be a difficulty for surface treatment processes typically involving higher
temperatures.
Finally, it should be emphasized that the models proposed above for lithium
storage in nongraphitic carbons are based on a limited number of experiments
made with a limited number of carbonaceous materials. This means that carbons
may be synthesized which do not belong to any of the above categories, that is,
hydrogen-containing carbon, soft or hard carbon, and so on. For example, it was
reported that milling [276] or strong burn-off [179, 249] of hard carbons in air alters
the charge–discharge curve completely, as these carbons show hysteresis, too.