Page 561 - Handbook of Battery Materials
P. 561
17.2 Components of the Liquid Electrolyte 535
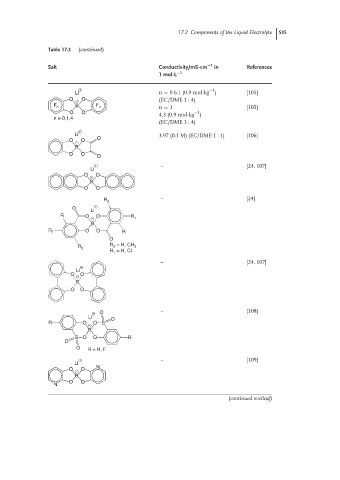
Table 17.3 (continued)
Salt Conductivity/mS·cm −1 in References
1 mol·L −1
+ −1
Li n = 06.1(0.9mol·kg ) [105]
O − O (EC/DME 1 : 4)
F n B F n n = 1 [105]
−1
O O 4.3 (0.9mol·kg )
n = 0,1,4
(EC/DME 1 : 4)
+
Li 3.97 (0.1 M) (EC/DME 1 : 1) [106]
O − O O
B
O O
O
+ – [24, 107]
Li
O − O
B
O O
R 2 – [24]
+
O
Li
R O O R
− 1
B
R 1 O O R
O
R R = H, CH 3
2
2
R = H, Cl
1
– [24, 107]
+
Li
O − O
B
O O
+ O – [108]
Li O
R O O S
−
B
S O O R
O
O R = H, F
+ – [109]
Li
N
O − O
B
O O
N
(continued overleaf)