Page 563 - Handbook of Battery Materials
P. 563
17.2 Components of the Liquid Electrolyte 537
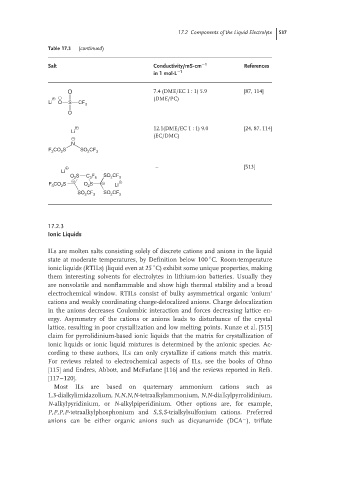
Table 17.3 (continued)
Salt Conductivity/mS·cm −1 References
in 1 mol·L −1
O 7.4 (DME/EC 1 : 1) 5.9 [87, 114]
+ − (DME/PC)
Li O S CF
3
O
+ 12.1(DME/EC 1 : 1) 9.0 [24, 87, 114]
Li
− (EC/DMC)
N
F CO S SO CF
3 2 2 3
+ – [513]
Li
S C F SO CF
O 2 3 6 2 3
− +
F CO S O S − Li
2
3
2
SO CF 3 SO CF 3
2
2
17.2.3
Ionic Liquids
ILs are molten salts consisting solely of discrete cations and anions in the liquid
◦
state at moderate temperatures, by Definition below 100 C. Room-temperature
◦
ionic liquids (RTILs) (liquid even at 25 C) exhibit some unique properties, making
them interesting solvents for electrolytes in lithium-ion batteries. Usually they
are nonvolatile and nonflammable and show high thermal stability and a broad
electrochemical window. RTILs consist of bulky asymmetrical organic ‘onium’
cations and weakly coordinating charge-delocalized anions. Charge delocalization
in the anions decreases Coulombic interaction and forces decreasing lattice en-
ergy. Asymmetry of the cations or anions leads to disturbance of the crystal
lattice, resulting in poor crystallization and low melting points. Kunze et al. [515]
claim for pyrrolidinium-based ionic liquids that the matrix for crystallization of
ionic liquids or ionic liquid mixtures is determined by the anionic species. Ac-
cording to these authors, ILs can only crystallize if cations match this matrix.
For reviews related to electrochemical aspects of ILs, see the books of Ohno
[115] and Endres, Abbott, and McFarlane [116] and the reviews reported in Refs.
[117–120].
Most ILs are based on quaternary ammonium cations such as
1,3-dialkylimidazolium, N,N,N,N-tetraalkylammonium, N,N-dialkylpyrrolidinium,
N-alkylpyridinium, or N-alkylpiperidinium. Other options are, for example,
P,P,P,P-tetraalkylphosphonium and S,S,S-trialkylsulfonium cations. Preferred
−
anions can be either organic anions such as dicyanamide (DCA ), triflate