Page 574 - Handbook of Battery Materials
P. 574
548 17 Liquid Nonaqueous Electrolytes
the FSI concentration and the magnitude of reversible current of lithium de-
position, but this coherence differs much for different anions [170]. ILs have
benefits as substitutes for solvents as they have a very low vapor pressure. They
can also be used as additives, e.g. Moosbauer et al. [255] did show that ad-
6
dition of ILs reduces the conductivity gap between LiPF and LiDFOB based
solutions.
17.2.4
Purification of Electrolytes
Purification of solvents and salts is an essential issue for reliable electrochemical
studies and measurements. A water content of 20 ppm already corresponds to a
10 −3 M solution. This is in the concentration range of dilute solutions used in
conductivity studies for the determination of association constants. Traces of water
may affect chemical equilibria and therefore act on specific conductivities and
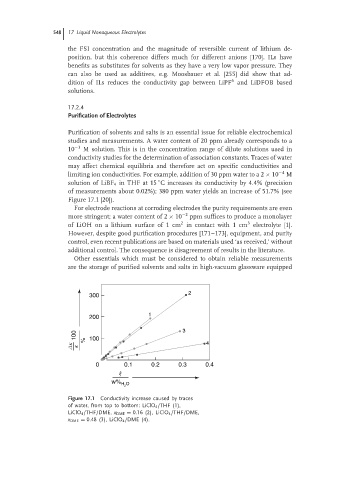
limiting ion conductivities. For example, addition of 30 ppm water to a 2 × 10 −4 M
◦
solution of LiBF 4 in THF at 15 C increases its conductivity by 4.4% (precision
of measurements about 0.02%); 380 ppm water yields an increase of 51.7% (see
Figure 17.1 [20]).
For electrode reactions at corroding electrodes the purity requirements are even
more stringent; a water content of 2 × 10 −2 ppm suffices to produce a monolayer
2
3
of LiOH on a lithium surface of 1 cm in contact with 1 cm electrolyte [1].
However, despite good purification procedures [171–173], equipment, and purity
control, even recent publications are based on materials used ‘as received,’ without
additional control. The consequence is disagreement of results in the literature.
Other essentials which must be considered to obtain reliable measurements
are the storage of purified solvents and salts in high-vacuum glassware equipped
2
300
200 1
3
100 % 100
Dk k 4
0 0.1 0.2 0.3 0.4
x
w% H O
2
Figure 17.1 Conductivity increase caused by traces
of water, from top to bottom: LiClO 4 /THF (1),
LiClO 4 /THF/DME, x DME = 0.16 (2), LiClO 4 /THF/DME,
x DME = 0.48 (3), LiClO 4 /DME (4).