Page 576 - Handbook of Battery Materials
P. 576
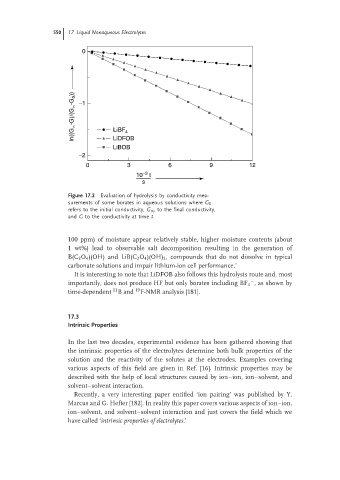
550 17 Liquid Nonaqueous Electrolytes
0
In((G ∝ -G)/(G ∝ -G 0 )) −1 LiBF 4
LiDFOB
LiBOB
−2
0 3 6 9 12
−3
10 t
s
Figure 17.2 Evaluation of hydrolysis by conductivity mea-
surements of some borates in aqueous solutions where G 0
refers to the initial conductivity, G ∞ to the final conductivity,
and G to the conductivity at time t.
100 ppm) of moisture appear relatively stable, higher moisture contents (about
1 wt%) lead to observable salt decomposition resulting in the generation of
B(C 2 O 4 )(OH) and LiB(C 2 O 4 )(OH) 2 , compounds that do not dissolve in typical
carbonate solutions and impair lithium-ion cell performance.’
It is interesting to note that LiDFOB also follows this hydrolysis route and, most
importantly, does not produce HF but only borates including BF 4 ,asshown by
−
time-dependent 11 B and 19 F-NMR analysis [181].
17.3
Intrinsic Properties
In the last two decades, experimental evidence has been gathered showing that
the intrinsic properties of the electrolytes determine both bulk properties of the
solution and the reactivity of the solutes at the electrodes. Examples covering
various aspects of this field are given in Ref. [16]. Intrinsic properties may be
described with the help of local structures caused by ion–ion, ion–solvent, and
solvent–solvent interaction.
Recently, a very interesting paper entitled ‘ion pairing’ was published by Y.
Marcus and G. Hefter [182]. In reality this paper covers various aspects of ion–ion,
ion–solvent, and solvent–solvent interaction and just covers the field which we
have called ‘intrinsic properties of electrolytes.’