Page 631 - Handbook of Battery Materials
P. 631
17.4 Bulk Properties 605
17.4.6.7 Transference numbers from NMR-diffusion coefficients
Transference numbers are defined as ratio of ion motion to electrolyte motion. One
possibility to calculate the transference number of a cation is the quotient of
diffusion coefficient of the cation divided by diffusion coefficient of cation plus
diffusion coefficient of anion:
D +
t + = (17.69)
D + + D −
The easiest way to determine D + and D − is the pfg-NMR method. The method has
the advantage that it is not restricted to binary electrolytes; even multicomponent
systems are realizable. However, it requires expensive instrumentation, is restricted
to NMR-visible nuclei, and cannot be compared to electrochemical methods,
because it averages ion pairs and isolated ions. In the literature, many examples of
these measurements are found [460–462], also for ILs [411, 463, 464]. Transference
numbers for lithium salts in IL as solvents have already been studied as well as
the improvement of the lithium-ion transference number by the choice of a useful
anion [411].
However, it should be stressed that battery electrolytes are often strongly as-
sociated. Therefore the results of this method for battery electrolytes should be
used with caution. Perhaps, by comparing results for model systems including
conductivity measurements, it will be possible to gain a correct interpretation of
values determined with this method.
17.4.6.8 Impedance Measurements
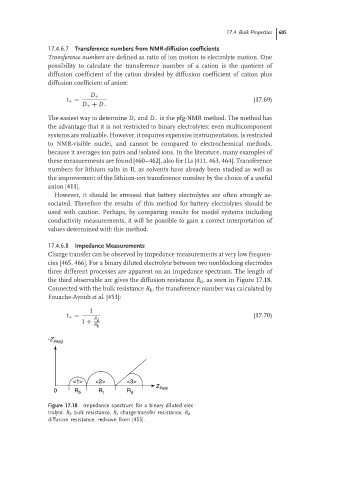
Charge transfer can be observed by impedance measurements at very low frequen-
cies [465, 466]. For a binary diluted electrolyte between two nonblocking electrodes
three different processes are apparent on an impedance spectrum. The length of
the third observable arc gives the diffusion resistance R d , as seen in Figure 17.18.
Connected with the bulk resistance R b , the transference number was calculated by
Fouache-Ayoub et al. [453]:
1
t + = (17.70)
R d
1 +
R
b
-Z Imag
<1> <2> <3>
Z
0 R b R t R d Real
Figure 17.18 Impedance spectrum for a binary diluted elec-
trolyte. R b bulk resistance, R t charge-transfer resistance, R d
diffusion resistance, redrawn from [453].