Page 636 - Handbook of Battery Materials
P. 636
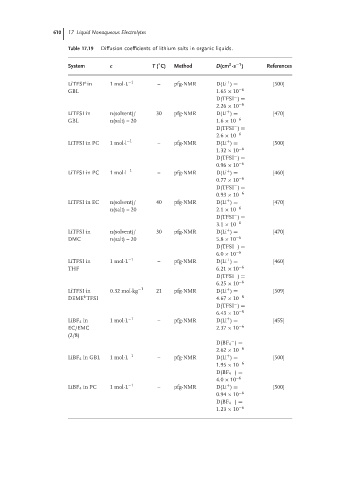
610 17 Liquid Nonaqueous Electrolytes
Table 17.19 Diffusion coefficients of lithium salts in organic liquids.
◦
2
−1
System c T ( C) Method D(cm ·s ) References
a
+
LiTFSI in 1mol·L −1 – pfg-NMR D(Li ) = [500]
GBL 1.65 × 10 −6
−
D(TFSI ) =
2.26 × 10 −6
+
LiTFSI in n(solvent)/ 30 pfg-NMR D(Li ) = [470]
GBL n(salt) = 20 1.6 × 10 −6
−
D(TFSI ) =
2.6 × 10 −6
+
LiTFSI in PC 1 mol·l −1 – pfg-NMR D(Li ) = [500]
1.32 × 10 −6
−
D(TFSI ) =
0.96 × 10 −6
LiTFSI in PC 1 mol·l −1 – pfg-NMR D(Li ) = [460]
+
0.77 × 10 −6
−
D(TFSI ) =
0.93 × 10 −6
LiTFSI in EC n(solvent)/ 40 pfg-NMR D(Li ) = [470]
+
n(salt) = 20 2.1 × 10 −6
−
D(TFSI ) =
3.1 × 10 −6
LiTFSI in n(solvent)/ 30 pfg-NMR D(Li ) = [470]
+
DMC n(salt) = 20 5.8 × 10 −6
−
D(TFSI ) =
6.0 × 10 −6
LiTFSI in 1mol·L −1 – pfg-NMR D(Li ) = [460]
+
THF 6.21 × 10 −6
−
D(TFSI ) =
6.25 × 10 −6
+
LiTFSI in 0.32 mol·kg −1 21 pfg-NMR D(Li ) = [509]
b
DEME TFSI 4.67 × 10 −8
−
D(TFSI ) =
6.43 × 10 −8
LiBF 4 in 1mol·L −1 – pfg-NMR D(Li ) = [455]
+
EC/EMC 2.37 × 10 −6
(2/8)
−
D(BF 4 ) =
2.62 × 10 −6
+
LiBF 4 in GBL 1 mol·L −1 – pfg-NMR D(Li ) = [500]
1.95 × 10 −6
−
D(BF 4 ) =
4.0 × 10 −6
+
LiBF 4 in PC 1 mol·L −1 – pfg-NMR D(Li ) = [500]
0.94 × 10 −6
−
D(BF 4 ) =
1.23 × 10 −6