Page 654 - Handbook of Battery Materials
P. 654
628 18 Polymer Electrolytes
• A plasticized electrolyte, usually obtained by the addition of small amounts of
a liquid of high dielectric constant to a solving polymer electrolyte in order to
enhance its conductivity.
• An ‘ionic rubber’ comprising a low-temperature molten salt mixture and a
small amount of high-molecular-weight polymer. On a structural level, these
electrolytes have some features in common with gel electrolytes. They were first
reported in the literature in 1993 [5] and are in the early stages of development.
• A membrane ionomer, in particular a polyelectrolyte with an inert backbone
such as Nafion . These require a plasticizer (typically water) to achieve good
conductivity levels and are associated primarily, in their proton-conducting form,
with solid polymer-electrolyte fuel cells.
The focus has largely been on poly-ether-based solvent-free systems for lithium
rechargeable batteries. They are simple to prepare, and their fundamental physical
and electrical properties are almost unique, making them interesting materials
for a broad range of fundamental research studies. More recently, studies have
been initiated on multivalent cation-based systems, with possible implications for,
amongst other things, calcium- and zinc-based electrochemical cells [6, 7]. Despite
the superior ionic conductivities that can be achieved with plasticized systems,
they have not had the same level of attention, mainly because they share many
properties and drawbacks of the liquid component, including those encountered
in cells containing metallic lithium electrodes.
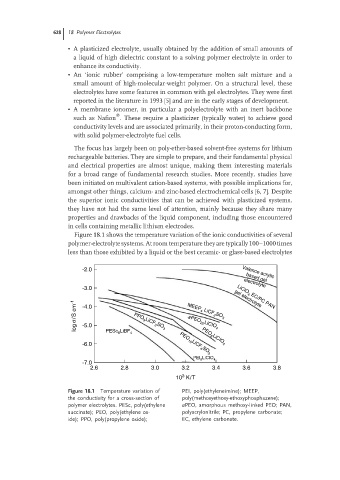
Figure 18.1 shows the temperature variation of the ionic conductivities of several
polymer-electrolyte systems. At room temperature they are typically 100–1000 times
less than those exhibited by a liquid or the best ceramic- or glass-based electrolytes
-2.0 Valence acrylic
based gel
electrolyte
-3.0 gel electrolyte
log σ/S cm -1 -4.0 PPO 9 LiCF 3 SO 3 MEEP 4 LiCF 3 SO 3
LiCIO 4 EC/PC PAN
-5.0
aPEO 20 LiCIO 4
6
-6.0 PESc LiBF 4 PEO 10 LiCF 4 SO 3
PEO 8 LiCIO 4
PEI LiCIO 4
8
-7.0
2.6 2.8 3.0 3.2 3.4 3.6 3.8
3
10 K/T
Figure 18.1 Temperature variation of PEI, poly(ethyleneimine); MEEP,
the conductivity for a cross-section of poly(methoxyethoxy-ethoxyphosphazene);
polymer electrolytes. PESc, poly(ethylene aPEO, amorphous methoxy-linked PEO; PAN,
succinate); PEO, poly(ethylene ox- polyacrylonitrile; PC, propylene carbonate;
ide); PPO, poly(propylene oxide); EC, ethylene carbonate.