Page 667 - Handbook of Battery Materials
P. 667
18.2 Solvent-Free Polymer Electrolytes 641
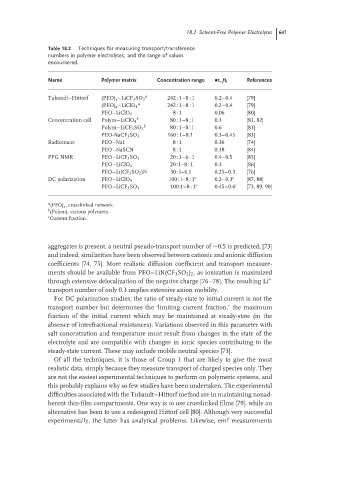
Table 18.2 Techniques for measuring transport/transference
numbers in polymer electrolytes, and the range of values
encountered.
Name Polymer matrix Concentration range •t + /t i References
Tubandt–Hittorf (PEO) x –LiCF 3 SO 3 a 242 : 1–8 : 1 0.2–0.4 [79]
a
(PEO) x –LiClO 4 242 : 1–8 : 1 0.2–0.4 [79]
PEO–LiClO 4 8 : 1 0.06 [80]
Concentration cell Polym–LiClO 4 b 80 : 1–8 : l 0.3 [81, 82]
b
Polym–LiCF 3 SO 3 80 : 1–8 : l 0.6 [83]
PEO-NaCF 3 SO 3 160 : 1–8.1 0.3–0.45 [83]
Radiotracer PEO–NaI 8 : 1 0.36 [74]
PEO–NaSCN 8 : 1 0.38 [84]
PFG NMR PEO–LiCF 3 SO 3 20 : 1–6 : 1 0.4–0.5 [85]
PEO–LiClO 4 20 : l–8 : 1 0.3 [86]
PEO–Li(CF 3 SO 2 )N 30 : l–6.1 0.25–0.3 [76]
DC polarization PEO–LiClO 4 100 : l–8 : 1 c 0.2–0.3 c [87, 88]
PEO–LiCF 3 SO 3 100:1–8 : 1 c 0.45–0.6 c [73, 89, 90]
a (PEO) x , crosslinked network.
b
(Polym), various polymers.
c Current fraction.
aggregates is present, a neutral pseudo-transport number of ∼0.5 is predicted, [73]
and indeed, similarities have been observed between cationic and anionic diffusion
coefficients [74, 75]. More realistic diffusion coefficient and transport measure-
ments should be available from PEO–LiN(CF 3 SO 2 ) 2 , as ionization is maximized
through extensive delocalization of the negative charge [76–78]. The resulting Li +
transport number of only 0.3 implies extensive anion mobility.
For DC polarization studies, the ratio of steady-state to initial current is not the
transport number but determines the ‘limiting current fraction,’ the maximum
fraction of the initial current which may be maintained at steady-state (in the
absence of interfractional resistances). Variations observed in this parameter with
salt concentration and temperature must result from changes in the state of the
electrolyte and are compatible with changes in ionic species contributing to the
steady-state current. These may include mobile neutral species [73].
Of all the techniques, it is those of Group 1 that are likely to give the most
realistic data, simply because they measure transport of charged species only. They
are not the easiest experimental techniques to perform on polymeric systems, and
this probably explains why so few studies have been undertaken. The experimental
difficulties associated with the Tubandt–Hittorf method are in maintaining nonad-
herent thin-film compartments. One way is to use crosslinked films [79], while an
alternative has been to use a redesigned Hittorf cell [80]. Although very successful
experimentally, the latter has analytical problems. Likewise, emf measurements