Page 668 - Handbook of Battery Materials
P. 668
642 18 Polymer Electrolytes
3
ZnCl 2 + CsCl
2
1 ZnCl 2
CF )
Zn(SO 3 3 2
i / nA 0
100 pA
−1
v = 4 mV.S -1
−2 T = 80°C
on Pt, ∅= 25 µm
−3
−0.2 0.0 0.2 0.4 0.6
2+
E / V vs. Zn /Zn
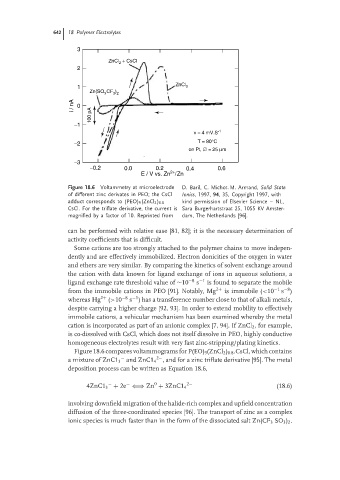
Figure 18.6 Voltammetry at microelectrode D. Baril, C. Michot. M. Armand, Solid State
of different zinc derivates in PEO; the CsCl Ionics, 1997, 94, 35, Copyright 1997, with
adduct corresponds to (PEO) 9 (ZnCl 2 ) 0.8 kind permission of Elsevier Science – NL,
CsCl. For the triflate derivative, the current is Sara Burgerhartstraat 25, 1055 KV Amster-
magnified by a factor of 10. Reprinted from dam, The Netherlands [96].
can be performed with relative ease [81, 82]; it is the necessary determination of
activity coefficients that is difficult.
Some cations are too strongly attached to the polymer chains to move indepen-
dently and are effectively immobilized. Electron donicities of the oxygen in water
and ethers are very similar. By comparing the kinetics of solvent exchange around
the cation with data known for ligand exchange of ions in aqueous solutions, a
ligand exchange rate threshold value of ∼10 −8 s −1 is found to separate the mobile
from the immobile cations in PEO [91]. Notably, Mg 2+ is immobile (<10 −1 −8
s )
s ) has a transference number close to that of alkali metals,
whereas Hg 2+ (>10 −8 −1
despite carrying a higher charge [92, 93]. In order to extend mobility to effectively
immobile cations, a vehicular mechanism has been examined whereby the metal
cation is incorporated as part of an anionic complex [7, 94]. If ZnCl 2 , for example,
is co-dissolved with CsCl, which does not itself dissolve in PEO, highly conductive
homogeneous electrolytes result with very fast zinc-stripping/plating kinetics.
Figure 18.6 compares voltammograms for P(EO) 9 (ZnCl 2 ) 0.8 .CsCl, which contains
− 2− , and for a zinc triflate derivative [95]. The metal
a mixture of ZnC1 3 and ZnC1 4
deposition process can be written as Equation 18.6,
− − 0 2−
4ZnC1 3 + 2e ⇐⇒ Zn + 3ZnC1 4 (18.6)
involving downfield migration of the halide-rich complex and upfield concentration
diffusion of the three-coordinated species [96]. The transport of zinc as a complex
ionic species is much faster than in the form of the dissociated salt Zn(CF 3 SO 3 ) 2 .