Page 673 - Handbook of Battery Materials
P. 673
18.3 Hybrid Electrolytes 647
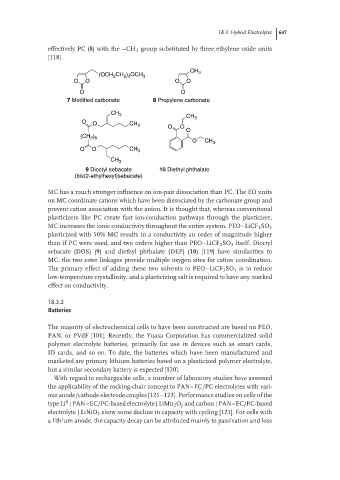
effectively PC (8) with the –CH 3 group substituted by three ethylene oxide units
[118].
CH 3
(OCH CH ) OCH 3
2 3
2
O O O O
O O
7 Modified carbonate 8 Propylene carbonate
CH 3 CH
O O CH 3 3
O
O
O
(CH 2 ) 8
O CH 3
O O CH 3
CH 3
9 Dioctyl sebacate 10 Diethyl phthalate
(bis(2-ethylhexyl)sebacate)
MC has a much stronger influence on ion-pair dissociation than PC. The EO units
on MC coordinate cations which have been dissociated by the carbonate group and
prevent cation association with the anion. It is thought that, whereas conventional
plasticizers like PC create fast ion-conduction pathways through the plasticizer,
MC increases the ionic conductivity throughout the entire system. PEO–LiCF 3 SO 3
plasticized with 50% MC results in a conductivity an order of magnitude higher
than if PC were used, and two orders higher than PEO–LiCF 3 SO 3 itself. Dioctyl
sebacate (DOS) (9) and diethyl phthalate (DEP) (10) [119] have similarities to
MC: the two ester linkages provide multiple oxygen sites for cation coordination.
The primary effect of adding these two solvents to PEO–LiCF 3 SO 3 is to reduce
low-temperature crystallinity, and a plasticizing salt is required to have any marked
effect on conductivity.
18.3.2
Batteries
The majority of electrochemical cells to have been constructed are based on PEO,
PAN, or PVdF [101]. Recently, the Yuasa Corporation has commercialized solid
polymer electrolyte batteries, primarily for use in devices such as smart cards,
ID cards, and so on. To date, the batteries which have been manufactured and
marketed are primary lithium batteries based on a plasticized polymer electrolyte,
but a similar secondary battery is expected [120].
With regard to rechargeable cells, a number of laboratory studies have assessed
the applicability of the rocking-chair concept to PAN–EC/PC electrolytes with vari-
ous anode/cathode electrode couples [121–123]. Performance studies on cells of the
0
type Li | PAN–EC/PC-based electrolyte | LiMn 2 O y and carbon | PAN–EC/PC-based
electrolyte | LiNiO 2 show some decline in capacity with cycling [121]. For cells with
a lithium anode, the capacity decay can be attributed mainly to passivation and loss