Page 674 - Handbook of Battery Materials
P. 674
648 18 Polymer Electrolytes
of lithium by its reaction with the electrolyte. A carbon/LiNiO 2 cell retains 85%
of the initial discharge capacity after >300 cycles. Reversibility can be improved
by replacing carbon with TiS 2 [122]. The reduction in capacity results from a rise
in internal impedance possibly associated with a reduction of the electrolyte on
carbon. Other factors such as solvent cointercalation, which in known to contribute
to the decline in capacity of similar organic-liquid-electrolyte-based cells, could also
be involved.
The preparation and properties of a novel, commercially viable Li-ion battery
based on a gel electrolyte has recently been disclosed by Bellcore (USA) [124].
The technology has, to date, been licensed to six companies, and full commercial
production is imminent. The polymer membrane is a copolymer based on PVdF
co-polymerized with hexafluoropropylene (HFP). HFP helps to decrease the crys-
tallinity of the PVdF component, enhancing its ability to absorb liquid. Optimizing
the liquid absorption ability, mechanical strength, and processability requires opti-
mized amorphous/crystalline-phase distribution. The PVdF–HFP membrane can
absorb plasticizer up to 200% of its original volume, especially when a pore former
(fumed silica) is added. The liquid electrolyte is typically a solution of LiPF 6 in
2 : 1 EC:dimethyl carbonate. A graphite carbon anode is used in conjunction
with a lithium manganese oxide cathode. Cell assembly is crucial to the final cell
performance. After the cell laminate has been processed, the membrane process-
ing plasticizer is extracted and replaced by the electrolyte solution to activate the
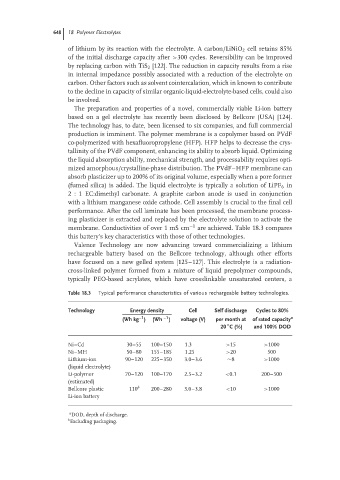
membrane. Conductivities of over 1 mS cm −1 are achieved. Table 18.3 compares
this battery’s key characteristics with those of other technologies.
Valence Technology are now advancing toward commercializing a lithium
rechargeable battery based on the Bellcore technology, although other efforts
have focused on a new gelled system [125–127]. This electrolyte is a radiation-
cross-linked polymer formed from a mixture of liquid prepolymer compounds,
typically PEO-based acrylates, which have crosslinkable unsaturated centers, a
Table 18.3 Typical performance characteristics of various rechargeable battery technologies.
Technology Energy density Cell Self discharge Cycles to 80%
–1
(Wh kg ) (Wh –1 ) voltage (V) per month at of rated capacity a
◦
20 C (%) and 100% DOD
Ni–Cd 30–55 100–150 1.3 >15 >1000
Ni–MH 50–80 155–185 1.25 >20 500
Lithium-ion 90–120 225–350 3.0–3.6 ∼8 >1000
(liquid electrolyte)
Li-polymer 70–120 100–170 2.5–3.2 <0.1 200–500
(estimated)
Bellcore plastic 110 b 200–280 3.0–3.8 <10 >1000
Li-ion battery
a DOD, depth of discharge.
b
Excluding packaging.