Page 59 - Handbook of Materials Failure Analysis
P. 59
1 Introduction 53
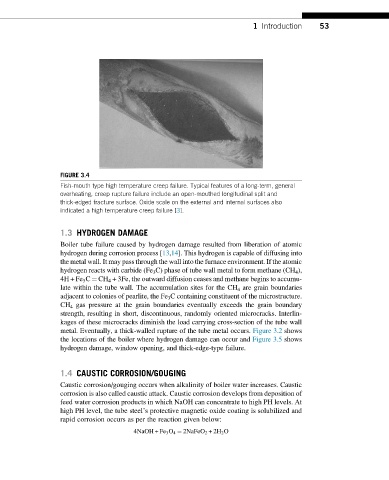
FIGURE 3.4
Fish-mouth type high temperature creep failure. Typical features of a long-term, general
overheating, creep rupture failure include an open-mouthed longitudinal split and
thick-edged fracture surface. Oxide scale on the external and internal surfaces also
indicated a high temperature creep failure [3].
1.3 HYDROGEN DAMAGE
Boiler tube failure caused by hydrogen damage resulted from liberation of atomic
hydrogen during corrosion process [13,14]. This hydrogen is capable of diffusing into
the metal wall. It may pass through the wall into the furnace environment. If the atomic
hydrogen reacts with carbide (Fe 3 C) phase of tube wall metal to form methane (CH 4 ),
4H + Fe 3 C ¼ CH 4 + 3Fe, the outward diffusion ceases and methane begins to accumu-
late within the tube wall. The accumulation sites for the CH 4 are grain boundaries
adjacent to colonies of pearlite, the Fe 3 C containing constituent of the microstructure.
CH 4 gas pressure at the grain boundaries eventually exceeds the grain boundary
strength, resulting in short, discontinuous, randomly oriented microcracks. Interlin-
kages of these microcracks diminish the load carrying cross-section of the tube wall
metal. Eventually, a thick-walled rupture of the tube metal occurs. Figure 3.2 shows
the locations of the boiler where hydrogen damage can occur and Figure 3.5 shows
hydrogen damage, window opening, and thick-edge-type failure.
1.4 CAUSTIC CORROSION/GOUGING
Caustic corrosion/gouging occurs when alkalinity of boiler water increases. Caustic
corrosion is also called caustic attack. Caustic corrosion develops from deposition of
feed water corrosion products in which NaOH can concentrate to high PH levels. At
high PH level, the tube steel’s protective magnetic oxide coating is solubilized and
rapid corrosion occurs as per the reaction given below:
4NaOH + Fe 3 O 4 ¼ 2NaFeO 2 +2H 2 O