Page 383 - Handbook of Properties of Textile and Technical Fibres
P. 383
356 Handbook of Properties of Textile and Technical Fibres
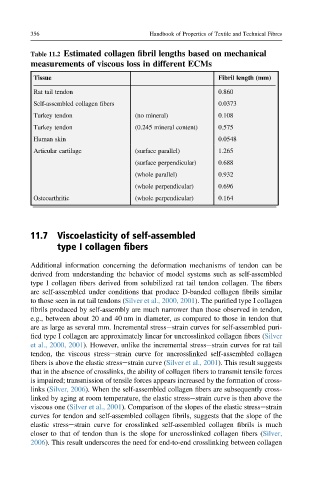
Table 11.2 Estimated collagen fibril lengths based on mechanical
measurements of viscous loss in different ECMs
Tissue Fibril length (mm)
Rat tail tendon 0.860
Self-assembled collagen fibers 0.0373
Turkey tendon (no mineral) 0.108
Turkey tendon (0.245 mineral content) 0.575
Human skin 0.0548
Articular cartilage (surface parallel) 1.265
(surface perpendicular) 0.688
(whole parallel) 0.932
(whole perpendicular) 0.696
Osteoarthritic (whole perpendicular) 0.164
11.7 Viscoelasticity of self-assembled
type I collagen fibers
Additional information concerning the deformation mechanisms of tendon can be
derived from understanding the behavior of model systems such as self-assembled
type I collagen fibers derived from solubilized rat tail tendon collagen. The fibers
are self-assembled under conditions that produce D-banded collagen fibrils similar
to those seen in rat tail tendons (Silver et al., 2000, 2001). The purified type I collagen
fibrils produced by self-assembly are much narrower than those observed in tendon,
e.g., between about 20 and 40 nm in diameter, as compared to those in tendon that
are as large as several mm. Incremental stressestrain curves for self-assembled puri-
fied type I collagen are approximately linear for uncrosslinked collagen fibers (Silver
et al., 2000, 2001). However, unlike the incremental stressestrain curves for rat tail
tendon, the viscous stressestrain curve for uncrosslinked self-assembled collagen
fibers is above the elastic stressestrain curve (Silver et al., 2001). This result suggests
that in the absence of crosslinks, the ability of collagen fibers to transmit tensile forces
is impaired; transmission of tensile forces appears increased by the formation of cross-
links (Silver, 2006). When the self-assembled collagen fibers are subsequently cross-
linked by aging at room temperature, the elastic stressestrain curve is then above the
viscous one (Silver et al., 2001). Comparison of the slopes of the elastic stressestrain
curves for tendon and self-assembled collagen fibrils, suggests that the slope of the
elastic stressestrain curve for crosslinked self-assembled collagen fibrils is much
closer to that of tendon than is the slope for uncrosslinked collagen fibers (Silver,
2006). This result underscores the need for end-to-end crosslinking between collagen