Page 104 - Handbook of Surface Improvement and Modification
P. 104
7.1 Methods and mechanisms of surface tension change 99
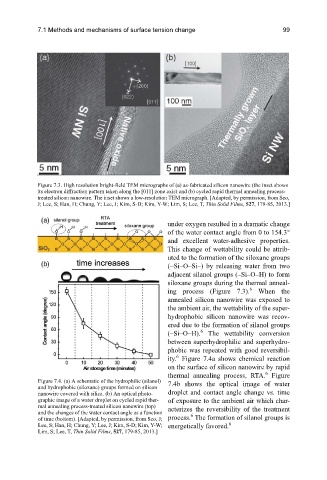
Figure 7.3. High resolution bright-field TEM micrographs of (a) as-fabricated silicon nanowire (the inset shows
its electron diffraction pattern taken along the [011] zone axis) and (b) cycled rapid thermal annealing process-
treated silicon nanowire. The inset shows a low-resolution TEM micrograph. [Adapted, by permission, from Seo,
J; Lee, S; Han, H; Chung, Y; Lee, J; Kim, S-D; Kim, Y-W; Lim, S; Lee, T, Thin Solid Films, 527, 179-85, 2013.]
under oxygen resulted in a dramatic change
of the water contact angle from 0 to 154.3°
and excellent water-adhesive properties.
This change of wettability could be attrib-
uted to the formation of the siloxane groups
(−Si–O–Si–) by releasing water from two
adjacent silanol groups (–Si–O–H) to form
siloxane groups during the thermal anneal-
6
ing process (Figure 7.3). When the
annealed silicon nanowire was exposed to
the ambient air, the wettability of the super-
hydrophobic silicon nanowire was recov-
ered due to the formation of silanol groups
6
(–Si–O–H). The wettability conversion
between superhydrophilic and superhydro-
phobic was repeated with good reversibil-
6
ity. Figure 7.4a shows chemical reaction
on the surface of silicon nanowire by rapid
6
thermal annealing process, RTA. Figure
Figure 7.4. (a) A schematic of the hydrophilic (silanol) 7.4b shows the optical image of water
and hydrophobic (siloxane) groups formed on silicon
nanowire covered with silica. (b) An optical photo- droplet and contact angle change vs. time
graphic image of a water droplet on cycled rapid ther- of exposure to the ambient air which char-
mal annealing process-treated silicon nanowire (top) acterizes the reversibility of the treatment
and the changes of the water contact angle as a function 6
of time (bottom). [Adapted, by permission, from Seo, J; process. The formation of silanol groups is
6
Lee, S; Han, H; Chung, Y; Lee, J; Kim, S-D; Kim, Y-W; energetically favored.
Lim, S; Lee, T, Thin Solid Films, 527, 179-85, 2013.]