Page 260 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 260
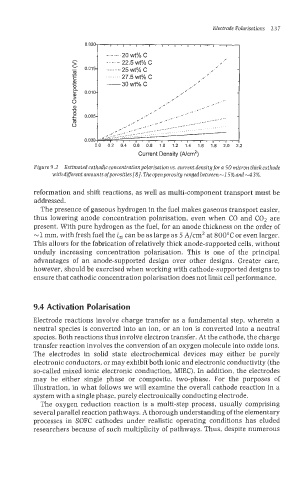
Electrode Polarisations 2 3 7
0.020- . , . ~ . , . , . , . , . , . , * , . , .
20 wt% c
,
h -. 22.5 wt% C ,
- ......... ,'
m
L 0.015-
.- 25 wt% C ,. ,'
c - /'
.a- ....... 27.5 wt% C
a,
+ 30 wt% C I'
,,'
0 ,
e 0.01c- 0-
!! .'
0'
,'
..-.
0 0. ,_..-
,,...-.
W
u .' 0' 0' _,._..-..-
2 0.005- C' .' 0. 0' .-..
m _..-. .......... ......... .........
_..-''
_'.I"
0 *. C' C' C' ..-'' .......... ........... ..........
.-..-
,. -..-. .......... ...............
,'
,,;,..;:::; .,.: ::., .....................
0.000,-;-"j'"'
reformation and shift reactions, as n7ell as multi-component transport must be
addressed.
The presence of gaseous hydrogen in the fuel makes gaseous transport easier,
thus lowering anode concentration polarisation, even when GO and GO2 are
present. With pure hydrogen as the fueI, for an anode thickness on the order of
--I mm, with fresh fuel the i,, can be as large as 5 A/cm2 at 800°C or even larger.
This allows for the fabrication of relatively thick anode-supported cells, without
unduly increasing concentration polarisation. This is one of the principal
advantages of an anode-supported design over other designs. Greater care,
however, should be exercised when working with cathode-supported designs to
ensure that cathodic concentration polarisation does not limit cell performance.
9.4 Activation Polarisation
Electrode reactions involve charge transfer as a fundamental step, wherein a
neutral species is converted into an ion, or an ion is converted into a neutral
species. Both reactions thus involve electron transfer. At the cathode, the charge
transfer reaction involves the conversion of an oxygen molecule into oxide ions.
The electrodes in solid state electrochemical devices may either be purely
electronic conductors, or may exhibit both ionic and electronic conductivity (the
so-called mixed ionic electronic conduction, MIEC). In addition, the electrodes
may be either single phase or composite, two-phase. For the purposes of
illustration, in what follows we will examine the overall cathode reaction in a
system with a single phase, purely eIectronically conducting electrode.
The oxygen reduction reaction is a multi-step process, usually comprising
several parallel reaction pathways. A thorough understanding of the elementary
processes in SOPC cathodes under realistic operating conditions has eluded
researchers because of such multiplicity of pathways. Thus, despite numerous