Page 265 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 265
242 High Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications
Gas Phase Gas Phase
0
oxygen .............. Oxygen Ion
Vacancy
Oxygen Vacancy
Ton
(a) fb)
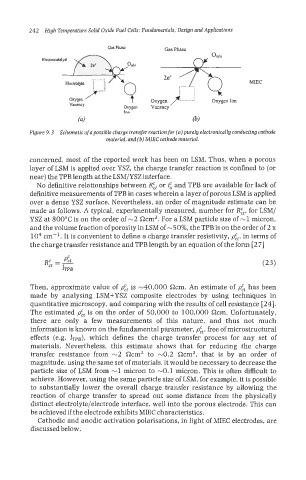
Figure 9.3 Schematic of apossible charge transfer reaction for (a) purely electronically conducting cathode
material, and (b) MlEC cathode materinl.
concerned, most of the reported work has been on LSM. Thus, when a porous
layer of LSM is applied over YSZ, the charge transfer reaction is confined to (or
near) the TPB length at the LSM/YSZ interface.
No definitive relationships between R:t or i: and TPB are available for lack of
definitive measurements of TPB in cases wherein a layer of porous LSM is applied
over a dense YSZ surface. Nevertheless, an order of magnitude estimate can be
made as follows. A typical, experimentally measured, number for R&, for LSM/
YSZ at 800°C is on the order of -2 Qcm2. For a LSM particle size of -1 micron,
and the volume fraction ofporosity in LSM of -SO%, the TPB is on the order of 2 x
lo4 cm-l. It is convenient to define a charge transfer resistivity, &, in terms of
the charge transfer resistance and TPB length by an equation of the form [2 71
Then, approximate value of p:t is -40,000 Qcm. An estimate of p:t has been
made by analysing LSM+YSZ composite electrodes by using techniques in
quantitative microscopy, and comparing with the results of cell resistance [24].
The estimated p:t is on the order of 50,000 to 100,000 Qcm. Unfortunately,
there are only a few measurements of this nature, and thus not much
information is known on the fundamental parameter, pEt, free of microstructural
effects (e.g. ITPB), which defines the charge transfer process for any set of
materials. Nevertheless, this estimate shows that for reducing the charge
transfer resistance from -2 Qcm2 to -0.2 Qcm2, that is by an order of
magnitude, using the same set of materials, it would be necessary to decrease the
particle size of LSM from -1 micron to ~0.1 micron. This is often difficult to
achieve. However, using the same particle size of LSM, for example, it is possible
to substantially lower the overall charge transfer resistance by allowing the
reaction of charge transfer to spread out some distance from the physically
distinct electrolyte/electrode interface, well into the porous electrode. This can
be achieved if the electrode exhibits MIEC characteristics.
Cathodic and anodic activation polarisations, in light of MIEC electrodes, are
discussed below.