Page 331 - High Temperature Solid Oxide Fuel Cells Fundamentals, Design and Applications
P. 331
Cell. Stack and System Modelling 307
Combining the above rate equations and mass balances with the flow and
thermal model equations presented in Section 11.2, detailed information about
the variation of gas composition, fuel or oxidant utilisation. etc., in the flow
channels may be generated.
The reactions presented thus far assume that the fuel mixture is CH4/H20. The
same approach would apply to other fuels such as Ha, CH30H, or dry CH4 with
corresponding changes in the reaction paths. If pure H2 is used with a small
amount of H20, the fuel composition and reaction mechanism are simplified. The
number of experimental parameters and mathematical equations needed is
reduced and the simulation is easier.
The equilibrium theory is very useful in addressing fuel processing issues
whether or not equilibrium is attained. For example, with an internal reforming
fuel cell the carbon forming reactions, decomposition of methane according to
CH4 -+ 2H2 + C, and Boudouard reaction, 2CO + C + C02, can be suppressed by
providing a proper molar ratio of water to methane. For the external reforming
subsystem, the theory can determine the optimal fuel-to-air ratio and operating
temperature to maximise stack fuel (H2 and CO) production while minimising
equilibrium-predicted carbon formation. The equilibrium theory can also guide
some cell design issues. Because steam reforming is an endothermic process,
excessive cooling of the stack at the fuel inlet can occur with internal reforming.
Nickel as an anode is known to be a good catalyst to promote cracking. A possible
improvement is kinetic suppression of the cracking reaction using catalysts that
are not as effective at promoting the cracking reaction. An alternative approach
would use catalysts that promote electrochemical oxidation of hydrocarbons at
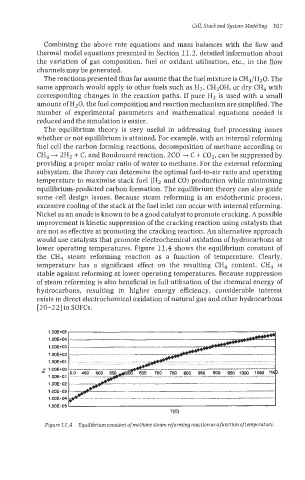
lower operating temperatures. Figure 11.4 shows the equilibrium constant of
the CH4 steam reforming reaction as a function of temperature. Clearly,
temperature has a significant effect on the resulting CH4 content. CH4 is
stable against reforming at lower operating temperatures. Because suppression
of steam reforming is also beneficial in full utilisation of the chemical energy of
hydrocarbons, resulting in higher energy efficiency, considerable interest
exists in direct electrochemical oxidation of natural gas and other hydrocarbons
[20-221 in SOFCs.
Figure 7 I .4 Equilibrium constant of methane steam reforming reaction as afunction of temperature.