Page 172 - Illustrated Pocket Dictionary of Chromatography
P. 172
174 SELECTIVITY
selectivity (1) A measure of how effectively a column/mobile
phase separates a pair of consecutively eluting peaks. Mathematically
the selectivity is often denoted as the separation factor, a, minus one:
a- 1. The selectivity is an explicit term in the determination of
the resolution between peaks, R s. (2) A detector that responds to a
particular class of compounds having a unique chemical or physical
property. An example is a UV detector that only responds to
molecules having a chromophore that absorbs at a set wavelength.
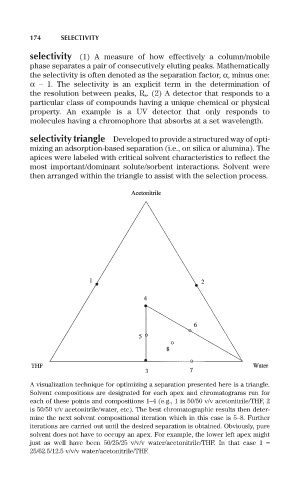
selectivity triangle Developed to provide a structured way of opti-
mizing an adsorption-based separation (i.e., on silica or alumina). The
apices were labeled with critical solvent characteristics to reflect the
most important/dominant solute/sorbent interactions. Solvent were
then arranged within the triangle to assist with the selection process.
A visualization technique for optimizing a separation presented here is a triangle.
Solvent compositions are designated for each apex and chromatograms run for
each of these points and compositions 1–4 (e.g., 1 is 50/50 v/v acetonitrile/THF, 2
is 50/50 v/v acetonitrile/water, etc). The best chromatographic results then deter-
mine the next solvent compositional iteration which in this case is 5–8. Further
iterations are carried out until the desired separation is obtained. Obviously, pure
solvent does not have to occupy an apex. For example, the lower left apex might
just as well have been 50/25/25 v/v/v water/acetonitrile/THF. In that case 1 =
25/62.5/12.5 v/v/v water/acetonitrile/THF.