Page 36 - Illustrated Pocket Dictionary of Chromatography
P. 36
CHIRAL STATIONARY PHASE (CSP) 31
chemisorption An adsorption process that results in an irrever-
sible chemical interaction of the analyte with the sorbent surface.
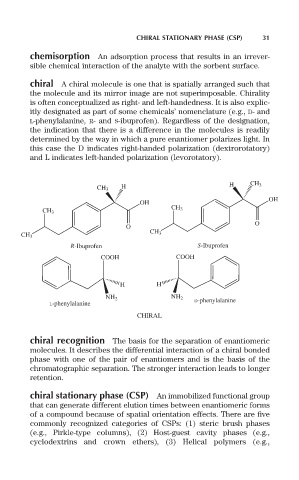
chiral A chiral molecule is one that is spatially arranged such that
the molecule and its mirror image are not superimposable. Chirality
is often conceptualized as right- and left-handedness. It is also explic-
itly designated as part of some chemicals’ nomenclature (e.g., D- and
L-phenylalanine, R- and S-ibuprofen). Regardless of the designation,
the indication that there is a difference in the molecules is readily
determined by the way in which a pure enantiomer polarizes light. In
this case the D indicates right-handed polarization (dextrorotatory)
and L indicates left-handed polarization (levorotatory).
H H CH 3
CH 3
OH
OH
CH 3
CH 3
O
O
CH 3
CH 3
R-Ibuprofen S-Ibuprofen
COOH COOH
H H
NH 2 NH 2 D-phenylalanine
L-phenylalanine
CHIRAL
chiral recognition The basis for the separation of enantiomeric
molecules. It describes the differential interaction of a chiral bonded
phase with one of the pair of enantiomers and is the basis of the
chromatographic separation. The stronger interaction leads to longer
retention.
chiral stationary phase (CSP) An immobilized functional group
that can generate different elution times between enantiomeric forms
of a compound because of spatial orientation effects. There are five
commonly recognized categories of CSPs: (1) steric brush phases
(e.g., Pirkle-type columns), (2) Host-guest cavity phases (e.g.,
cyclodextrins and crown ethers), (3) Helical polymers (e.g.,