Page 298 - Industrial Ventilation Design Guidebook
P. 298
254 CHAPTER 5 PHYSIOLOGICAL AND TOX1COLOGICAL CONSIDERATIONS
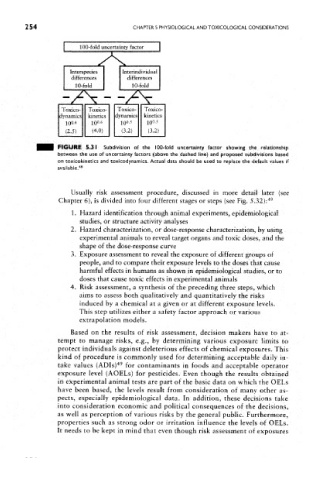
FIGURE 5.31 Subdivision of the 100-fold uncertainty factor showing the relationship
between the use of uncertainty factors (above the dashed line) and proposed subdivisions based
on toxicokinetics and toxicodynamics. Actual data should be used to replace the default values if
available. 48
Usually risk assessment procedure, discussed in more detail later (see
Chapter 6), is divided into four different stages or steps (see Fig. 5.32): 49
1. Hazard identification through animal experiments, epidemiological
studies, or structure activity analyses
2. Hazard characterization, or dose-response characterization, by using
experimental animals to reveal target organs and toxic doses, and the
shape of the dose-response curve
3. Exposure assessment to reveal the exposure of different groups of
people, and to compare their exposure levels to the doses that cause
harmful effects in humans as shown in epidemiological studies, or to
doses that cause toxic effects in experimental animals
4. Risk assessment, a synthesis of the preceding three steps, which
aims to assess both qualitatively and quantitatively the risks
induced by a chemical at a given or at different exposure levels.
This step utilizes either a safety factor approach or various
extrapolation models.
Based on the results of risk assessment, decision makers have to at-
tempt to manage risks, e.g., by determining various exposure limits to
protect individuals against deleterious effects of chemical exposures. This
kind of procedure is commonly used for determining acceptable daily in-
49
take values (ADIs) for contaminants in foods and acceptable operator
exposure level (AOELs) for pesticides. Even though the results obtained
in experimental animal tests are part of the basic data on which the OELs
have been based, the levels result from consideration of many other as-
pects, especially epidemiological data. In addition, these decisions take
into consideration economic and political consequences of the decisions,
is well as perception of various risks by the general public. Furthermore,
properties such as strong odor or irritation influence the levels of OELs.
tt needs to be kept in mind that even though risk assessment of exposures