Page 12 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 12
2 Smith
by
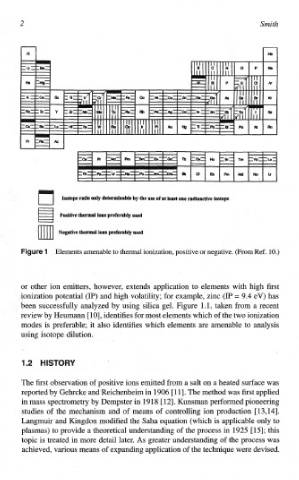
Isotope ratio only de~r~nable the use of at feast one radioactive isotope
Positive thermal ions preferably used
Negative thema1 ions preferabiy used
Elements amenable to thermal ionization, positive or negative. (From Ref. lo.)
or other ion emitters, however, extends application to elements with high first
* nization potential (IP) and high volatility; for example, zinc (IP = 9.4 eV) has
een successfully analyzed by using silica gel. Figure 1.1, taken from a recent
review by Heurnann [lo], identifies for most elements which of the two ionization
modes is preferable; it also identifies which elements are amenable to analysis
using isotope dilution.
The first observation of positive ions emitted from a salt on a heated surface was
reported by Cehcke and ~eichenheim in 1906 [ 1 11. The method was first applied
in mass spectrometry by Dempster in 191 8 [ 121. Kunsman performed pioneering
studies of the mechanism and of means of controlling ion production [13,14].
Langmuir and Kingdon modified the Saha equation (which is applicable only to
plasmas) to provide a theoretical understanding of the process in 1925 [ 151; this
topic is treated in more detail later. As greater understanding of the process was
achieved, various means of expanding application of the technique were devised.