Page 15 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 15
Thermal Ionization Mass Spe~tro~et~ 5
0 5 10
EXPOSURE (10" torr-sec)
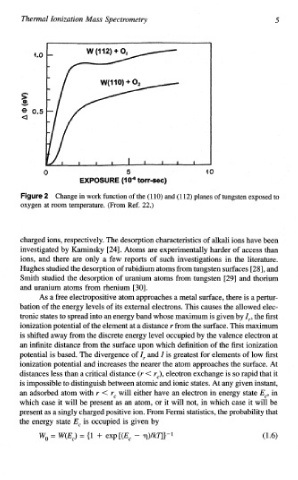
Figure 2 Change in work function of the (1 10) and (1 12) planes of tungsten exposed to
oxygen at room temperature. (From Ref. 22.)
charged ions, respectively. The desorption characteristics of alkali ions have been
investigated by Kaminsky [24]. Atoms are experimentally harder of access than
ions, and there are only a few reports of such investigations in the literature.
Hughes studied the desorption of rubidium atoms from tungsten surfaces [28], and
Smith studied the desorption of uranium atoms from tungsten [29] and thorium
and uranium atoms from rhenium [30].
As a free electropositive atom approaches a metal surface, there is a pertur-
bation of the energy levels of its external electrons. This causes the allowed elec-
tronic states to spread into an energy band whose maximum is given by Ir, the first
ionization potential of the element at a distance r from the surface. This m~imu~
is shifted away from the discrete energy level occupied by the valence electron at
an infinite distance from the surface upon which definition of the first ionization
potential is based. The divergence of Ir and I is greatest for elements of low first
ionization potential and increases the nearer the atom approaches the surface. At
distances less than a critical distance (r < rc), electron exchange is so rapid that it
is impossible to distinguish between atomic and ionic states. At any given instant,
an adsorbed atom with r < rc will either have an electron in energy state Ec, in
which case it will be present as an atom, or it will not, in which case it will be
present as a singly charged positive ion. From Fed statistics, the probability that
the energy state Ec is occupied is given by
IVo = W(Ec) = (1 + exp [(Ec - q)/kZ'J]-l (1.6)