Page 124 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 124
114 Olesik
50
2 40
h
v)-
uv)
c u
f 2 30
U ::S
3 .,g 20
SkL
.-
v) 10
0
0.9 1.0 1.1 1.2 1.3 1.4 0.9 1.0 1.1 1.2 1.3 1.4
Nebulizer gas flow rate (Llmin) Nebulizer gas flow rate (Llmin)
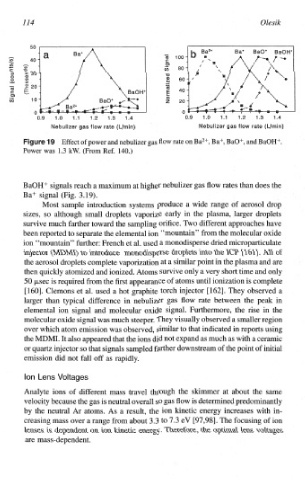
Effect of power and nebulizer gas Aow rate on Ba2+, Ba+, BaO+, and BaOH+.
Power was 1.3 kW. (From Ref. 140.)
BaOH” signals reach a maximum at higher nebulizer gas flow rates than does
the
Ba+ signal (Fig. 3.19).
Most sample introduction systems produce a wide range of aerosol drop
sizes, so although small droplets vaporize early in the plasma, larger droplets
survive much farther toward the sampling orifice. Two different approaches have
been reported to separate the elemental ion “mountain” from the molecular oxide
ion “mountain” further: French et al. used a monodisperse dried microparticulate
injector (M~~I) to introduce monodisperse droplets into the ICP [161]. All of
at
the aerosol droplets complete vaporization a similar point in the plasma and are
then quickly atomized and ionized. Atoms survive only
a very short time and only
50 psec is required from the first appearance of atoms until ionization is complete
[ 1601. Clemons et al. used a hot graphite torch injector [ 1621. They observed a
larger than typical difference in nebulizer gas flow rate between the peak in
elemental ion signal and molecular oxide signal. Furthermore, the rise in the
a
molecular oxide signal was much steeper. They visually observed smaller region
to
over which atom emission was observed, similar that indicated in reports using
the M~MI. It also appeared that the ions did not expand
as much as with a ceramic
or quartz injector so that signals sampled farther downstream of the point of initial
emission did not fall off as rapidly.
Ion Lens Voltages
Analyte ions of different mass travel through the skimmer at about the same
velocity because the gas
is neutral overall so gas flow is determined predominantly
by the neutral Ar atoms. As a result, the ion kinetic energy increases with in-
creasing mass over a range from about 3.3 to 7.3 eV [97,98]. The focusing of ion
lenses is dependent on ion kinetic energy. Therefore, the optimal lens voltages
are mass-dependent.