Page 316 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 316
~ultiple-Collect~r ICP-MS 303
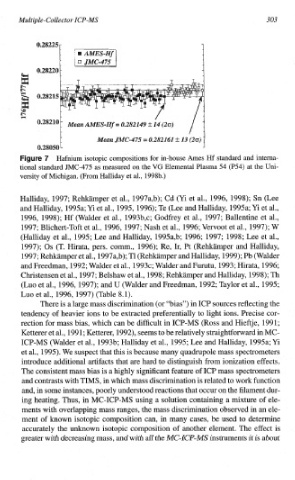
0.28225 r l
~
~ ~ = 0.282261 rt 13 -(20) ~ ~ 5
~~~~
0.2~~5Q
7 Hafnium isotopic compositions for in-house Ames Hf standard and interna-
tional standard JMC-475 as measured on the VC Elemental Plasma 54 (P54) at the Uni-
versity of Michigan. (From Halliday et al., 1998b.)
Halliday, 1997; Rehkamper et al., 1997a,b); Cd (Yi et al., 1996, 1998); Sn (Lee
and Halliday, 199%; Yi et al., 1995, 1996); Te (Lee and Halliday, 199%; Yi et al.,
1996, 1998); Hf (Walder et al., 1993b,c; Godfrey et al., 1997; Ballentine et al.,
1997; Blichert-Toft et al., 1996, 1997; Nash et al., 1996; Vewoot et al., 1997); W
(Halliday et al., 1995; Lee and Halliday, 1995a,b; 1996; 1997; 1998; Lee et al.,
1997); Os (T. Hirata, pers. comm., 1996); Re, Ir, Pt (Rehkamper and Halliday,
1997; Rehkamper et al., 1997a,b); Ti (Rehkamper and Halliday, 1999); Pb (Walder
and Freedman, 1992; Walder et al., 1993c; Walder and Furutu, 1993; Hirata, 1996;
Christensen et al., 1997; elshaw et al., 1998; RehkWper and Halliday, 1998); Th
(Luo et al., 1996, 1997); and U (Walder and Freedman, 1992; Taylor et al., 1995;
Luo et al., 1996, 1997) (Table 8.1).
There is a large mass discri~nation (or “bias”) in ICP sources reflecting the
tendency of heavier ions to be extracted preferentially to light ions. Precise cor-
rection for mass bias, which can be difficult in ICP-MS (Ross and Hieftje, 1991;
Ketterer et al., 1991; Ketterer, 1992), seems to be relatively str~ghtfo~~d in MC-
ICP-MS (Walder et al., 1993b; Halliday et al., 1995; Lee and Halliday, 199%; Yi
et al., 1995). We suspect that this is because many quadrupole mass spectrometers
introduce additional artifacts that are hard to distinguish from ionization effects.
The consistent mass bias is a highly signi~cant feature of ICP mass spectrometers
and contrasts with TIMS, in which mass disc~mination is related to work function
dur-
and, in some instances, poorly understood reactions that occur on the filament
ing heating. Thus, in ~C-ICP-MS using a solution containing a mixture of ele-
ments with overlapping mass ranges, the mass discri~nation observed in an ele-
ment of known isotopic composition can, in many cases, be used to determine
accurately the unknown isotopic composition of another element. The effect is
greater with decreasing mass, and with
all the MC-ICP-MS instruments it is about