Page 44 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 44
potential between the negatively biased cathode and the anode. Free electrons in
the gas are accelerated toward the positively biased anode by the resulting poten-
tial gradient. These free electrons collide with gaseous species, producing gas-
phase ions. Positive ions are accelerated toward and impinge on the negatively
biased electrode. On impact, a variety of secondary species are liberated, includ-
ing electrons and species formed from the cathode material. The ions and atoms
from the cathode have analytical utility since they represent the sample. The entire
process is considered self-sustaining on the basis that electrons are created at
the cathode to replace those lost at the anode. Hence, no external ionization is
required [25].
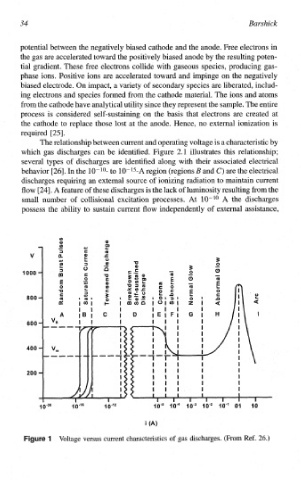
The relationship between current and operating voltage is a characteristic by
which gas discharges can be identified. Figure 2.1 illustrates this relationship;
several types of discharges are identified along with their associated electrical
behavior [26]. In the to 10-15-A region (regions B and C) are the electrical
discharges requiring an external source of ionizing radiation to maintain current
flow [24]. A feature of these discharges is the lack of luminosity resulting from the
small number of collisional excitation processes. At 10-lo A the discharges
possess the ability to sustain current flow independently of external assistance,
0
- _.
0
0
-
0
m
5
0 ’
ZI
GI(
600 I
I
I
I
00 I
I I l l I I ’
200 I I l l I I
I 1 1 1 I I
I 1 1 1 I I
I I I
1 o-20 1 o”2 lo-* lo-’ 01 10
Voltage versus current characteristics of gas discharges. (From Ref. 26.)