Page 48 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 48
38
Sputtered Particle
Vacuu~
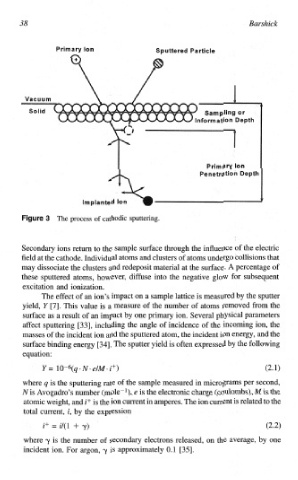
The process of cathodic sputtering.
Secondary ions return to the sample surface through the influence of the electric
field at the cathode. Individual atoms and clusters of atoms undergo collisions that
may dissociate the clusters and redeposit material at the surface. A percentage of
these sputtered atoms, however, diffuse into the negative glow for subsequent
excitation and ionization.
The eEect of an ion’s impact on a sample lattice is measured by the sputter
yield, Y [7]. This value is a measure of the number of atoms removed from the
surface as a result of an impact by one primary ion. Several physical parameters
afTect sputtering [33], including the angle of incidence of the incoming ion, the
masses of the incident ion and the sputtered atom, the incident ion energy, and the
surface binding energy [34]. The sputter yield is often expressed by the following
equation:
Y = 10-6(q .N. e/M. if) (2.1)
where q is the sputtering rate of the sample measured in micrograms per second,
N is Avogadro’s number (mole-l), e is the electronic charge (coulombs), M is the
atomic weight, and i+ is the ion current in amperes. The ion current is related to the
total current, i, by the expression
i+ = i/(l + y) (2.2)
where y is the number of secondary electrons released, on the average, by one
incident ion. For argon, y is approximately 0.1 [35].