Page 51 - Inorganic Mass Spectrometry - Fundamentals and Applications
P. 51
Glow Discharge Mass Spectrometry
Electron impact (El) ionization
X0
enning (metasta~l~) ionization
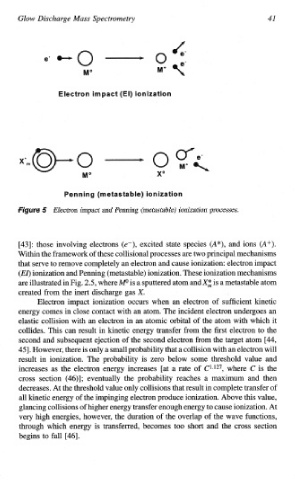
Figure 5 Electron impact and Penning (metastable) ionization processes.
[43]: those involving electrons (e-), excited state species (A*), and ions (A+).
Within the framework of these collisional processes are two principal mechanisms
that serve to remove completely an electron and cause ionization: electron impact
(EZ) ionization and Penning (metastable) ionization. These ionization mechanisms
are illustrated in Fig. 2.5, where W is a sputtered atom and X; is a metastable atom
created from the inert discharge gas X.
Electron impact ionization occurs when an electron of sufficient kinetic
energy comes in close contact with an atom. The incident electron undergoes an
elastic collision with an electron in an atomic orbital of the atom with which it
collides. This can result in kinetic energy transfer from the first electron to the
second and subsequent ejection of the second electron from the target atom [44,
451. However, there is only a small probability that collision with an electron will
a
result in ionization. The probability is zero below some threshold value and
increases as the electron energy increases [at a rate of where C is the
cross section (46)]; eventually the probability reaches a maximum and then
decreases. At the threshold value only collisions that result complete transfer of
in
all kinetic energy of the impinging electron produce ionization. Above this value,
glancing collisions of higher energy transfer enough energy to cause ionization. At
very high energies, however, the duration of the overlap of the wave functions,
through which energy is transferred, becomes too short and the cross section
begins to fall [46].