Page 67 - Inorganic Mass Spectrometry : Fundamentals and Applications
P. 67
Glow Discharge Mass Spectro~et~ S7
Zirconium, aluminum, lithium, and boron are all important elements in
nuclear chemistry [loll; zirconium alloys, for example, are used as cladding for
nuclear fuel. Because nuclear processes distort the isotopic composition of these
elements, measuring them before and after a process provides important informa-
tion with regard to reactor operation. Glow discharge mass spectrometry is well
suited to this type of measurement, as exemplified by the isotopic analysis of zir-
conium samples containing plutonium [ 1011. It had been proposed that the anal-
ysis of zirconium alloys could be hindered by the presence of multiply charged
isobaric interferences formed though the combination of plutonium with argon
[ 1011. No evidence of the formation of the plutonium argide bivalent and trivalent
species was found, however, and the isotopic composition of zirconiu~ in an
[ 1011.
unknown sample compared well with the composition of natural zirconium
the
In many applications, discerning the importance abundance of a particu-
lar isotope is difficult without a precise measurement. Experiments at the Oak
Ridge National Laboratory were directed at establishing how well isotope ratios
could be measured with GDMS. External precision was better than 0.03% for
ratios measured for the matrix element [ 1021. When the element was present in
concentrations of -0.5 weight percent, external precision was better than 0.1 5%;
this value worsened to 1% for elements with concentrations in the 10- to 20-ppm
range [102]. Although these ratios are of sufficient quality for many applications,
some results suggest that better values would have been obtained by better
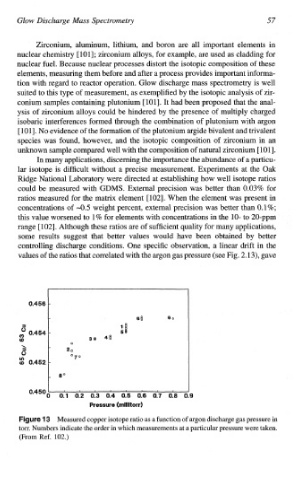
controlling discharge conditions. One specific observation, a linear drift in the
values of the ratios that correlated with the argon gas pressure (see Fig. 2.13), gave
t 8o
Measured copper isotope ratio as a function of argon discharge gas pressure in
torr. Numbers indicate the order in which measurements at a particular pressure were taken.
(From Ret 102.)