Page 200 - Instant notes
P. 200
Physical chemistry 186
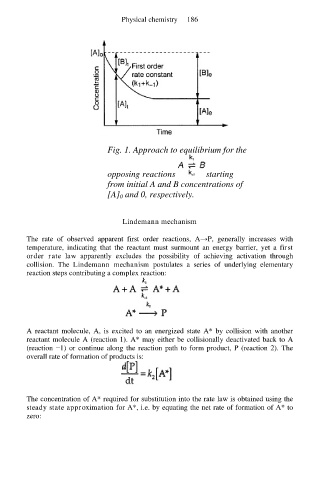
Fig. 1. Approach to equilibrium for the
opposing reactions starting
from initial A and B concentrations of
[A] 0 and 0, respectively.
Lindemann mechanism
The rate of observed apparent first order reactions, A→P, generally increases with
temperature, indicating that the reactant must surmount an energy barrier, yet a first
order rate law apparently excludes the possibility of achieving activation through
collision. The Lindemann mechanism postulates a series of underlying elementary
reaction steps contributing a complex reaction:
A reactant molecule, A, is excited to an energized state A* by collision with another
reactant molecule A (reaction 1). A* may either be collisionally deactivated back to A
(reaction −1) or continue along the reaction path to form product, P (reaction 2). The
overall rate of formation of products is:
The concentration of A* required for substitution into the rate law is obtained using the
steady state approximation for A*, i.e. by equating the net rate of formation of A* to
zero: